
Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma and other liver diseases
Introduction
Hepatocellular carcinoma (HCC) is considered as the fifth most common cancer and the third main cause of cancer-related deaths (1,2). The prognoses of HCC patients remain bleak, even though there have been significant advancements in surgical techniques, liver transplantation, local-regional treatments, and molecular target therapy (3). Owing to the interplay of tumor development and liver function, the complexity in managing HCC is dramatically increased. Clinical practice and outcomes are both significantly influenced by the underlying liver dysfunction, as well as by the stage and extent of the tumor itself (4).
For liver function assessment, the Child-Pugh (CP) score is used by most of the international guidelines for the management of HCC. Furthermore, CP score is adopted into inclusion and exclusion criteria in the majority of clinical trials for HCC (5-10). The CP score is calculated from five variables: three liver function tests (total bilirubin, albumin and prothrombin time); and two based on clinical symptoms (ascites and hepatic encephalopathy). However, as essential as the conventional CP classification is for the assessment of liver function, its imperfections introduce a number of limitations.
Firstly, the CP score was originally developed, using an empirical rather than evidence-based method, for assessing the prognoses of patients affected by cirrhosis and portal hypertension who undergo surgical treatment for variceal bleeding (11,12). In this sense, CP score is more suitable to be applied in cirrhotic rather than non-cirrhotic patients. Clinically, however, it is clear that, of HCC patients, a significant proportion have a non-cirrhotic background (13,14). According to reports, there was no histological evidence of cirrhosis in almost half of patients who undergo surgical resection of HCC (15). Secondly, it includes subjective factors evaluating the presence of ascites and encephalopathy. A consensus on how to determine the severity of these symptoms is yet to be agreed. For example, the grade of the severity of ascites may depend on a physician’s experience. Furthermore, ascites may also be affected by the diuretic or by the tumor itself. An equal amount of difficulty may be encountered when grading encephalopathy, as many of its early symptoms resemble those of the HCC itself. Thirdly, laboratory variables, which include albumin, bilirubin and prothrombin time have their cut-off points calculated arbitrarily. Fourthly, there is close interrelation between some of the indexes in the CP class, including ascites and serum albumin. Fifthly, as most HCC patients were found to be CP A at diagnosis, it has been suggested that for the assessment of liver function, CP score shows a relative lack of granularity (16).
With regard to the limitations of CP score outlined above, the albumin-bilirubin (ALBI) grade, a novel nomogram of liver function assessment sorely based on serum albumin and total bilirubin, has recently been proposed as an alternative for HCC patients (16). The ALBI grade can objectively stratify HCC patients into three risk groups and predict significantly different overall survival (OS) during follow-up. In comparison with CP score, it has three main advantages. Firstly, it was established through an evidence-based process especially for the assessment of liver function in HCC patients, and—in both non-cirrhotic and cirrhotic populations—performs adequately. Secondly, as it solely uses the criteria of serum albumin and total bilirubin, it is a simple and objective method of assessment which removes the need for subjective clinical variables and makes the classification between observers more reliable. Thirdly, it has a higher granularity than CP score for assessing liver function because it can stratify CP A patients according to different survival outcomes (17).
The prognostic value of ALBI grade in HCC after different therapies
Many studies have validated the ALBI grade’s prognostic power in different therapies and HCC staging systems (Table 1). Baseline ALBI grade has been shown in many studies to be a powerful prognostic factor for OS, no matter what the therapeutic option is (4,18-25). Furthermore, with an increase of ALBI grade, the OS of patients becomes significantly worse. In the study by Kuo et al., the OS for ALBI grades 1, 2 and 3 after sorafenib therapy significantly decreased from 14.8 months, to 5 and 0.7 months, respectively (18). Similarly, in the study by Pinato et al., the survival outcomes of ALBI grade 1, 2 and 3 were 13, 6.3 and 1.6 months, respectively (19). In addition, the hazard ratio (HR) between ALBI 2 and 1 was 1.7 (95% CI: 1.1–2.9) and between ALBI 3 and ALBI 1 was 4.1 (95% CI: 2.5–6.8). In the study by Kuo et al., those with increased ALBI grade had significantly poorer survival than those with non-changed ALBI grade after sorafenib treatment (2.1 vs. 6.4 months, P<0.001) (18).
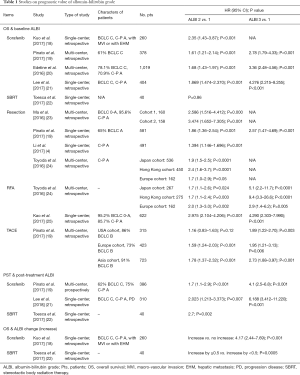
Full table
Association of ALBI grade and BCLC stage
As the Barcelona Clinic Liver Cancer (BCLC) staging system is one of the most commonly used HCC staging systems, the prognostic power of the ALBI grade across each of the BCLC stages has been the focus of a large number of studies.
Using a stage-stratified approach, Pinato et al. investigated the ALBI grade’s prognostic ability in both curative and palliative groups and proved it has sufficient and clinically significant stratifying power across all of the BCLC stages of HCC (17). In early-stage HCC, they confirmed that the ALBI grade could distinguish those with poorer prognosis within CP A class patients, who are generally regarded as candidates for surgical resection (26). Li et al. (4) confirmed that there can be increased survival outcomes for patients undergoing resection at ALBI grade 1 in comparison with those at ALBI grade 2 in the BCLC stage 0-A (68 vs. 52 months, P=0.004) and stage B (26 vs. 20 months, P=0.044). In intermediate-stage HCC, it was reported that the ALBI grade was an accurate predictor of survival for patients treated with transcatheter arterial chemoembolization (TACE) in three geographically distinct patient cohorts (the USA, Europe, and Asia cohorts). In advanced HCC, the study showed that, in addition to its ability to predict survival independently, the ALBI grade outperformed CP in identifying patients with a higher risk of early mortality before sorafenib initiation.
Incorporating ALBI grade into different HCC staging systems
A patient’s clinical stage is the principal factor in the clinical management of their HCC. In addition to the BCLC staging system, there have been several other clinical staging systems proposed for HCC, including the American Joint Committee on Cancer (AJCC) staging system, the Okuda classification, the Cancer of the Liver Italian Program (CLIP), and the Japan Integrated Staging (JIS) score (27-31). However, the ability of current ongoing staging systems to accurately predict prognoses and inform guiding treatment selection remains inadequate. For example, a significant proportion of BCLC patients, particularly those in East Asia, undergo hepatectomy, which BCLC guidelines recommend for patients at BCLC stage 0-A (32). Many BCLC stage A and C patients also receive TACE therapy, which BCLC guidelines recommend for BCLC stage B patients. This phenomenon may be largely connected with inaccurate assessment of liver function.
The newly established ALBI grade has been incorporated into some of the HCC staging systems and has successfully replaced the original liver function evaluation method used in HCC staging systems, producing a better prognostic power according to homogeneity likelihood Chi-Square, C-Index, and corrected Akaike information criterion (AIC). Chan et al.’s study explored the prognostic ability of the CP-based BCLC system in comparison with the ALBI-based BCLC system and demonstrated a similar overall prognostic performance between the two systems with regard to homogeneity, discriminatory ability, and the monotonicity of gradients (33). Shao et al.’s study proved ALBI-CLIP had higher R and lower AIC than CLIP, demonstrating ALBI-CLIP might perform better than CLIP did in predicting prognosis (34).
There were two studies that compared the prognostic ability of the CP-based JIS score with that of the ALBI-based JIS score (ALBI-T score) (35,36). The results of these studies also indicated that the ALBI-T score performed better than the CP-based JIS score in predicting prognosis. A further two studies that compared the prognostic performances of JIS score, liver damage base JIS score (modified JIS score) and ALBI-T score. In the study by Hiraoka et al., AIC results showed that the prognostic performance of the ALBI-T score was better than modified JIS score and JIS score (37).
Prognostic comparison of ALBI grade and CP class
The comparisons between the prognostic values of ALBI grade and CP class are shown in Table 2. Although some studies which only compared one of the indexes were not shown in the table, most studies showed ALBI grade to have better discriminatory abilities than CP score. In contrast with most other studies, Edeline et al. (20) found that ALBI grade shared a similar prognostic power with CP score for CP A group (AICs: 6,649 vs. 6,652, P=0.02, Harrel’s C-Index: 0.56 vs. 0.57), although CP score showed better discriminatory ability for patients overall (AICs: 8,876 vs. 8,898, P<0.001). For trials of systemic therapy, their findings placed CP class A within the included criteria but found ALBI to be only a stratification indicator. There were also studies comparing ALBI grade with liver damage grade (LD), which is a more effective indicator than CP score for patients with early-stage HCC (38). Hiraoka et al. demonstrated that despite LD slightly outperforming ALBI grade in terms of AIC value for patients with early-stage HCC who received RFA, the median survival time for each individual ALBI grade exceeded that of the corresponding LD (39).
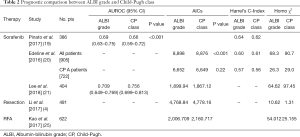
Full table
ALBI grade in CP class A patients
CP A patients are usually considered candidates for a variety of HCC treatments. For example, CP A class patients are generally regarded as ideal candidates for surgical resection (26). International guidelines suggest that TACE should be limited to those with CP A disease. Current guidelines and prospective trials restrict the use of stereotactic body radiation therapy (SBRT) to patients with CP class A cirrhosis (40-42) due to concerns regarding liver tolerance to radiation. Previous studies usually applied CP class A or B7 as their eligibility criteria (6,7,17,18,20,21). However, it is recognized that, within this single grade, there is a wide variation in hepatic function (43-45). Of the studies included in Table 3, many showed ALBI to improve the accuracy of prognoses, especially within the CP A group (46). These studies demonstrated that although patients were all classified as CP A, their prognoses still varied a lot, and they could be sub-classified into different grades according to ALBI grade with significantly different survival outcomes. Due to the disadvantages of CP score and the advantages of ALBI grade previously mentioned, as well as the obvious survival heterogeneity within CP A, ALBI grade may be more suitable than CP score as one of the inclusion criteria in relation to liver function assessment in HCC patients. No comparisons of the subclassifications by points [5–15] within the CP classification were included in the original study. Some studies compared the prognostic power of ALBI 1/2 and CP 5/6. Most of them determined that ALBI 1/2 had a superior differentiating efficacy for prognoses than C-P 5/6 within the Child Pugh (CP) A class.
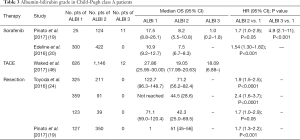
Full table
Refinement of ALBI grade: ALBI 2A and 2B
Although ALBI grade has been proved to improve prognostic power, there remain some drawbacks. Some studies have suggested a potentially higher prognostic heterogeneity in grade 2 patients. For example, Pinato et al. found that, while 92% of patients in ALBI grade 1 met the criteria of CP A, the percentage of patients in ALBI grades 2 and 3 who satisfied the criteria was lower (73% and 3% of ALBI grade 2 and 3, respectively) (18). Similarly, in Ogasawara et al.’s study, the majority of patients with ALBI grade 1 had a CP score of 5, in contrast with patients with ALBI grade 2, who had a wide range of liver reserve according to the CP score, with scores of 5, 6, 7, and ≥8 (47). ALBI grade 2 patients were divided into two groups, ALBI grade 2A and 2B, according to the median ALBI 2 score (grade 2A, ≤−2.118 and grade 2B, ≥2.118). Unlike most studies, the results demonstrated that there was no significant difference in prognosis for ABLI grades 1 and 2, while prognosis of ALBI grade 2B group was significantly worse than that of the ALBI grade 1 group (P=0.029) and of the 2A group (P=0.004). According to the multivariate survival analyses, the risk of death was particularly higher in ALBI grade 2B group (HR, 2.265; 95% CI, 1.021–5.022; P=0.044). Thus, it was suggested that the subdivision of patients with ALBI grade 2 could increase the efficiency of ALBI in distinguishing the prognoses of patients receiving sorafenib treatment, which may be suitable for patients who are in the advanced stages of HCC and ALBI grade 1, and for some with ALBI grade 2 (notably ALBI 2A). Considering the small number of studies on the subdivision of ALBI 2, more studies are required to validate these results.
Other systems based on ALBI grade
MICAN
BCLC guidelines recommend TACE to intermediate-stage HCC patients (48). However, hepatic function, as well as tumor size and number beyond Milan criteria (multinodular HCC without macrovascular invasion or extrahepatic metastasis) all inform the prognoses of intermediate-stage HCC patients (38,42,49-53). Based on clinical manifestation, Japanese clinical practice guidelines recommend a variety of therapies, such as surgical resection (hepatectomy) and TACE, for intermediate-stage HCC patients (54-57). Recently, a Japanese study (58) proposed a new sub-staging method, called Modified Intermediate Stage of Liver Cancer (MICAN) criteria, which predicts intermediate-stage patients’ prognoses and informs their treatment using the ALBI grade. Four sub-stages were defined by ALBI grade and up-to-7 criteria: ALBI-1/within up-to-7 criteria (B1), ALBI-2/within up-to-7 criteria (B2), ALBI-1 and ALBI-2/multiple and beyond up-to-7 criteria (B3), and ALBI-3/any (B4) (31). The median survival time for those classified as B1, B2, B3, and B4 was 65.1, 48.1, 29.6, and 14.6 months, respectively (P<0.01 for each comparison). As the MICAN stage increased, the availability of curative treatments like hepatectomy and RFA decreased, while the availability of palliative treatments like TACE and sorafenib increased. For instance, 67.0% of those in the B1 sub-stage underwent hepatectomy and received RFA, with the percentages of those in the B2, B3 and B4 sub-stages receiving the same treatments falling to 51.4%, 28.3% and 12.1%, respectively. The study also compared MICAN criteria with other evaluation systems which use subclassification to inform prognoses, such as Bolondi’s criteria and Kinki criteria and concluded that the result of the AIC for the MICAN criteria was superior to those of Bolondi’s and the Kinki criteria (990.5, 993.0 and 1,001.4, respectively) (59,60). The study confirmed that MICAN criteria based on ALBI grade can be applied for predicting survival and guiding therapies in the heterogeneous population of BCLC-B patients (58).
Platelet-bilirubin-albumin grade
Although the ALBI grade has been widely validated, it fails to incorporate portal hypertension. The platelet-bilirubin-albumin (PALBI) grade, which incorporates the blood platelet count as a surrogate marker for portal hypertension, was produced through additional modifications to the ALBI grade (61).
Liu et al.’s study found there to be a significant difference in survival across all of the ALBI and PALBI grades (62). Both grades could predict survival across various HCC stages, yet the PALBI grade’s AUC was significantly higher than the ALBI grade’s at 1, 3, and 5 years. Based on current studies, more research is required to determine whether or not the PALBI grade has better prognostic power than the ALBI grade.
ALBI grade and progression disease patterns
Those advanced-stage HCC patients who had failed first-line therapy but who had better liver reserves were potentially eligible for second-line clinical trials (62,63). Earlier second-line trials typically included CP class A or B7 in their eligibility criteria (64-68). However, as previously mentioned, there is heterogeneity in survival outcomes for CP A patients.
Lee et al. further proposed the novel ALBI grade and progression disease (ALBI-PD) criteria for second-line trials (21). The study considered ALBI grade 3 at PD, new extrahepatic lesions (NEH), and early PD within 4 months to be independent predictors of poor post-progression survival, and these were then incorporated into the ALBI-PD criteria. Of the patients in this study classified as having CP A liver function at PD, those who met the ALBI-PD criteria had significantly longer median PPS than patients who were beyond it. Furthermore, for those with CP A5 to B7 status, ALBI-PD criteria could significantly discriminate the PPS. They also compared the ALBI-PD criteria with the ALBI grade and conventional CP class. According to ROC analysis, ALBI-PD score better predicted post-progression mortality at 6, 9, and 12 months. The ALBI-PD criteria also performed well with regard to having the lowest AIC value and highest homogeneity (presented by Wald χ2 value).
ALBI grade in other liver diseases
Due to the accurate discriminative power of the ALBI grade in liver function evaluation, several studies have validated the performance of the ALBI grade in other liver diseases besides HCC.
Toesca et al. demonstrated a correlation between baseline ALBI grade (P=0.02) and post-treatment ALBI (HR: 2.9, P=0.013) and a worse OS time in cholangiocarcinoma patients treated with SBRT (22). In relation to patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure, Chen and Lin confirmed the prognostic ability of the ALBI score in predicting 3-month outcomes (69). Chen et al. demonstrated the higher accuracy of the ALBI score—in comparison to the CP and model for end-stage liver disease (MELD) scores—when used to predict long-term prognoses for patients with HBV-related cirrhosis (70). Chan et al. confirmed that ALBI grade was an independent factor in predicting the prognoses of patients with primary biliary cirrhosis and, when compared to alternative prognostic evaluation systems, gave a better or comparable performance (71).
Conclusions
Overall, the ALBI grade’s significant superiority in HCC and other liver diseases has been recognized. Many studies have confirmed that baseline ALBI grade is an important prognostic factor for OS. However, more studies are needed to evaluate the prognostic value of post-treatment ALBI grade and changes in ALBI grade. In addition, there is still some debate surrounding the superiority of ALBI grade over the conventional CP score. More high-level evidence is required to investigate this issue and to assist in establishing better HCC staging systems based on ALBI grade.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81702999), the Innovation Capability Support Program of Shaanxi province (2018KJXX-076) and the Health and Family Planning Commission of Shaanxi province (2017SF-208) for LL.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.116). LL serves as the unpaid editorial board member of Annals of Translational Medicine from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008 Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat 2009;16:453-63. [Crossref] [PubMed]
- Li MX, Zhao H, Bi XY, et al. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma: Validation in a Chinese cohort. Hepatol Res 2017;47:731-41. [Crossref] [PubMed]
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293-300. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med. 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013;31:4067-75. [Crossref] [PubMed]
- Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-24. [Crossref] [PubMed]
- Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol 2015;33:172-9. [Crossref] [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1-85. [PubMed]
- Chan AW, Chong CC, Mo FK, et al. Incorporating albumin-bilirubin grade into the cancer of the liver Italian program system for hepatocellular carcinoma. J Gastroenterol Hepatol 2017;32:221-8. [Crossref] [PubMed]
- van Meer S, van Erpecum KJ, Sprengers D, et al. Hepatocellular carcinoma in cirrhotic versus noncirrhotic livers: results from a large cohort in the Netherlands. Eur J Gastroenterol Hepatol 2016;28:352-9. [Crossref] [PubMed]
- Chan AW, Yu S, Yu YH, et al. Steatotic hepatocellular carcinoma: a variant associated with metabolic factors and late tumour relapse. Histopathology 2016;69:971-84. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. [Crossref] [PubMed]
- Kuo YH, Wang JH, Hung CH, et al. Albumin-Bilirubin grade predicts prognosis of HCC patients with sorafenib use. J Gastroenterol Hepatol 2017;32:1975-81. [Crossref] [PubMed]
- Pinato DJ, Yen C, Bettinger D, et al. The albumin-bilirubin grade improves hepatic reserve estimation post-sorafenib failure: implications for drug development. Aliment Pharmacol Ther 2017;45:714-22. [Crossref] [PubMed]
- Edeline J, Blanc JF, Johnson P, et al. A multicentre comparison between Child Pugh and Albumin-Bilirubin scores in patients treated with sorafenib for Hepatocellular Carcinoma. Liver Int 2016;36:1821-8. [Crossref] [PubMed]
- Lee PC, Chen YT, Chao Y. Validation of the ALBI Grade-based Integrated Model as a Predictor for Sorafenib-failed Hepatocellular Carcinoma. Liver Int 2018;38:321-30. [Crossref] [PubMed]
- Toesca DAS, Osmundson EC, von Eyben R, et al. Assessment of hepatic function decline after stereotactic body radiation therapy for primary liver cancer. Pract Radiat Oncol 2017;7:173-82. [Crossref] [PubMed]
- Ma XL, Zhou JY, Gao XH, et al. Application of the albumin-bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinoma. Clin Chim Acta 2016;462:15-22. [Crossref] [PubMed]
- Toyoda H, Lai PB, O'Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744-50. [Crossref] [PubMed]
- Kao WY, Su CW, Chiou YY, et al. Hepatocellular Carcinoma: Nomograms Based on the Albumin-Bilirubin Grade to Assess the Outcomes of Radiofrequency Ablation. Radiology 2017;285:670-80. [Crossref] [PubMed]
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis 2005;25:181-200. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918-28. [Crossref] [PubMed]
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [Crossref] [PubMed]
- Kudo M, Chung H, Osaki Y, et al. Prognostic staging system for hepatocellular carcinoma CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score JIS score. J Gastroenterol 2003;38:207-15. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Chan AW, Kumada T, Toyoda H, et al. Integration of albumin-bilirubin ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1300-6. [Crossref] [PubMed]
- Shao YY, Liu TH, Lee YH, et al. Modified CLIP with objective liver reserve assessment retains prognosis prediction for patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1336-41. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1031-6. [Crossref] [PubMed]
- Chan AW, Chong CC, Mo FK, et al. Applicability of albumin-bilirubin-based Japan integrated staging score in hepatitis B-associated hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1766-72. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Kudo M, et al. Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer 2017;6:204-15. [Crossref] [PubMed]
- Omagari K, Ohba K, Kadokawa Y, et al. Comparison of the grade evaluated by "Liver damage" of Liver Cancer Study Group of Japan and Child-Pugh classification in patients with hepatocellular carcinoma. Hepatol Res 2006;34:266-72. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Hirooka M, et al. A better method for assessment of hepatic function in hepatocellular carcinoma patients treated with radiofrequency ablation: Usefulness of albumin-bilirubin grade. Hepatol Res 2018;48:E61-7. [Crossref] [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Verslype C, Rosmorduc O, Rougier P, et al. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii41-8. [Crossref] [PubMed]
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Schneider PD. Preoperative assessment of liver function. Surg Clin North Am 2004;84:355-73. [Crossref] [PubMed]
- Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res 2009;39:107-16. [Crossref] [PubMed]
- Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Sci 2010;17:380-4. [Crossref] [PubMed]
- Waked I, Berhane S, Toyoda H, et al. Transarterial chemo-embolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br J Cancer 2017;116:448-54. [Crossref] [PubMed]
- Ogasawara S, Chiba T, Ooka Y, et al. Liver function assessment according to the Albumin-Bilirubin ALBI) grade in sorafenib-treated patients with advanced hepatocellular carcinoma. Invest New Drugs 2015;33:1257-62. [Crossref] [PubMed]
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. [Crossref] [PubMed]
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [Crossref] [PubMed]
- Reig M, Darnell A, Forner A, et al. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin Liver Dis 2014;34:444-55. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Piscaglia F, Bolondi L, et al. The intermediate hepatocellular carcinoma stage: Should treatment be expanded? Dig Liver Dis 2010;42:S258-63. [Crossref] [PubMed]
- Daoudaki M, Fouzas I. Fouzas, Hepatocellular carcinoma. Wiener Medizinische Wochenschrift 2014;164:450-5. [Crossref] [PubMed]
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology JSH) 2010 updated version. Dig Dis 2011;29:339-64. [Crossref] [PubMed]
- Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol 2012;56:886-92. [Crossref] [PubMed]
- Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J Gastroenterol 2006;12:828-9. [Crossref] [PubMed]
- Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000;32:1224-9. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Nouso K, et al. Proposed New Sub-Grouping for Intermediate-Stage Hepatocellular Carcinoma Using Albumin-Bilirubin Grade. Oncology 2016;91:153-61. [Crossref] [PubMed]
- Kudo M, Arizumi T, Ueshima K, et al. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification Kinki Criteria. Dig Dis 2015;33:751-8. [Crossref] [PubMed]
- Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012;32:348-59. [PubMed]
- Ni JY, Fang ZT, An C, et al. Comparison of albumin-bilirubin grade, platelet-albumin-bilirubin grade and Child-Turcotte-Pugh class for prediction of survival in patients with large hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Int J Hyperthermia 2019;36:841-53. [Crossref] [PubMed]
- Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol 2017;32:879-86. [Crossref] [PubMed]
- Shao YY, Wu CH, Lu LC, et al. Prognosis of patients with advanced hepatocellular carcinoma who failed first-line systemic therapy. J Hepatol 2014;60:313-8. [Crossref] [PubMed]
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-16. [Crossref] [PubMed]
- Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol 2013;14:55-63. [Crossref] [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Chen B, Lin S. Albumin-bilirubin (ALBI) score at admission predicts possible outcomes in patients with acute-on-chronic liver failure. Medicine (Baltimore) 2017;96:e7142. [Crossref] [PubMed]
- Chen RC, Cai YJ, Wu JM, et al. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis. J Viral Hepat 2017;24:238-45. [Crossref] [PubMed]
- Chan AW, Chan RC, Wong GL, et al. New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score. J Gastroenterol Hepatol 2015;30:1391-6. [Crossref] [PubMed]


