
Skeletal growth velocity of adolescent idiopathic scoliosis: abnormal in spine but normal in lower limbs
Introduction
Adolescent idiopathic scoliosis (AIS) is a 3-dimensional spine deformity that influences patient appearance with body disfigurement and causes pulmonary function impairment (1,2). To date, the specific pathogenesis of AIS remains unclear (3,4). A range of growth abnormalities have been identified in AIS patients throughout the pubertal growth period, including higher corrected height and lower body mass index (BMI) compared to healthy adolescents (5,6). It has been proposed that scoliosis might be caused by a discrepancy between the growth velocity of the anterior and posterior columns of the spine (7). However, recent studies have shown that anterior overgrowth might be due to an adaptation to the altered loading, rather than a primary growth disorder in the spine (8,9).
While the spinal overgrowth has been confirmed in AIS patients, the lower limbs have received little attention from spine surgeons and researchers alike. To date, only a few studies investigated the growth pattern of the lower limbs in patients with AIS. Dimeglio et al. (10) suggested that the peak height velocity (PHV) in patients with AIS is not a single time point, but rather a combination of three micro-peaks during puberty involving first the growth of lower limbs followed by the growth of the spine, and later the broadening of the thorax. However, the study did not clarify whether the growth disturbance seen in AIS patients is only localized at the spine or is a phenomenon that affects the whole skeleton, including the lower limbs. In addition, the study solely relied on anthropometric measurements, and a more accurate and quantitative assessment is warranted. With recent advances in radiological equipment, the new full-body length imaging system allows the visualization of the body from head to ankle in its actual size and proportions (11), providing a quantified method to evaluate the exact lengths of the lower limbs and their relationships with abnormal spinal growth in patients with AIS.
The prospective research aimed to investigate the PHV time point of lower limbs between AIS and healthy adolescents and to clarify whether there is the abnormal growth of the lower limbs in AIS patients.
Methods
Cohort
The ethics committee of our hospital approved this prospective study; 73 female adolescent AIS patients with thoracic curve were enrolled between November 2016 and July 2017. The inclusion criteria included patients with a main thoracic curve (the Cobb angle between 20° and 60°); a thoracic vertebral rotation(Nash-Moe grade I) (12); normal thoracic kyphosis (the angle between the superior endplate of T4 and the inferior endplate of T12 ranging between 10° and 40°); with full-body X-ray images; and without any prior treatment. Exclusion criteria were as follows: patients with current or a history of hip joint diseases; patients with a history of back pain, hip or lower limb discrepancy or disease; patients with spondylolisthesis. Another 42 age-matched control subjects were selected from a prospective database containing more than 300 asymptomatic subjects with full-body films who were recruited when attending a clinic with no or minor spinal asymmetry.
Measurements on coronal films
All images were acquired using a full-body imaging system (EOS® imaging, Paris, France) (11,13). During the procedure, patients received standardized verbal instructions by radiographers to “keep the eyes horizontal and look straight ahead, stand straight without leaning forwards or backwards, and touch the collar bones with the fingers.”
Full-body coronal films of the spine, pelvis, and lower limbs were obtained from all participants. Using the PACS system (Picture Archiving and Communication System, GE healthcare, Mount Prospect, IL, USA), the following parameters were obtained (Figure 1):
- Length of the spine (LOS) (line A). The midpoint of the superior endplate of each vertebra was selected, and straight lines connected every two points. The LOS was defined as the sum of the lengths of straight lines from T1 to S1.
- Height of the pelvis (HOP) (line B). A horizontal line was passed through the upper margin of the ilium and another through the ischial tuberosity. The offset between the two lines was defined as the HOP.
- Length of the lower limbs (LLL) (lines C+D). Line C was drawn between the greater trochanter and the center of the notch. Line D was drawn between the middle of the tibial spine and the middle of the distal articular surface of the tibia. LLL was defined as the sum of the lengths corresponding to line C and line D.
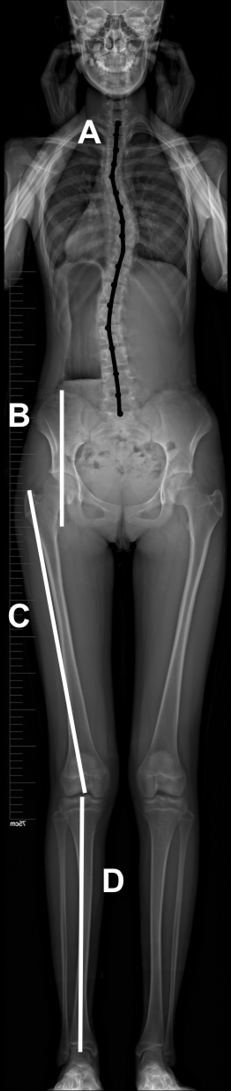
All patients and controls were classified into three groups according to skeleton maturity status (14): pre-PHV, defined as Risser 0, and open triradiate cartilage (TC); during-PHV (Risser 0, and closed TC); and post-PHV (Risser ranging from 1 to 5). All subjects were also divided into two groups according to Risser grades, with group A including AIS and healthy girls at Risser grade 0, and group B including AIS and healthy girls at Risser grade 4 and 5.
Standardized ratios were used to investigate growth features in subjects while minimizing the effect of personal differences. RatioSL was defined as LOS/LLL. Similarly, RatioSP was defined as LOS/HOP and the RatioPL as HOP/LLL. The ratios were calculated to evaluate better the growth of the spine and lower limbs among the cohort using the self-control method. All parameters and ratios were compared between AIS and control group, and all the ratios were compared between these three maturity stages.
All the parameter measurements were performed by two independent surgeons (SS and TZ) to assess inter-observer reliability. Furthermore, another researcher (QG) repeated the measurements after half a month to compare intra-observer reliability.
Statistical analysis
Data were analyzed with SPSS 23.0 (SPSS Inc., Chicago, IL). The independent t test was used to compare continuous parameters between AIS patients and healthy adolescents in group A and group B. Each dependent variable was compared between the three skeleton maturity groups (pre-PHV, during-PHV, and post-PHV groups) with ANOVA analysis. A P value less than 0.05 was considered statistically significant.
Results
The demographic characteristics of all subjects are shown in Table 1. The mean age of patients with AIS was 13.6±2.55 years (range: 10 to 18 years), similarly to the age of the control group (12.9±2.36 years, P=0.174). The mean magnitude of the main thoracic curve was 30.5°±12.03° (range: 20° to 59°). In the group of AIS patients, 46 subjects (63.01%) were post-PHV, versus 26 subjects (61.90%) in the control group. The reliability analysis showed high intra- and inter-observer agreements in the evaluation of parameters, with intraclass correlations (ICC) more than 0.8 (Table 2).

Full table
Full table
The differences in parameters and ratios in Group A (Risser 0) and Group B (Risser ≥4) are summarized in Table 3. No significant differences were found in LOS, HOP, or LLL between the two groups (all P>0.1). In order to eliminate the effect of individual variance, the standardized ratios were also compared between the subgroups. The RatioSL and RatioSP were significantly higher in patients with AIS at Risser ≥4 compared to healthy controls (P=0.033, and 0.003, respectively), implying that the final length of the spine was longer in patients with AIS. Meanwhile, RatioPL had no significant differences between the patients with AIS and controls in both the Risser 0 and Risser ≥4 groups (P=0.499 and 0.303, respectively). Besides, no significant differences were found in 3 ratios between patients with AIS and controls with Risser 0 (P=0.780, 0.367, and 0.499, respectively). These results indicated that the final spinal length was longer in AIS patients compared to that of healthy girls, while the length of the lower extremities was similar.

Full table
Moreover, the ratios were compared within the subgroups according to maturity status (Table 4). The change of these ratios from the pre-PHV to the post-PHV showed the similar trends in both scoliosis patients and controls: RatioSL and RatioPL were significantly the lowest in the during-PHV group (all P<0.02), implying PHVs of the spine and the pelvis are later than PHV of the lower extremities. RatioSP showed no significant differences between the three maturity subgroups in the control group (P=0.884). Meanwhile, RatioSP was higher in the post-period of PHV in the AIS group (P=0.003), revealing abnormal spinal overgrowth in patients with AIS.

Full table
Discussion
Numerous studies have reported that the spinal length of AIS patients is longer than that of controls (8,15,16). However, to date, no study has solely focused on the growth pattern of the lower extremities in AIS patients. The present study demonstrated that the final length of the lower limbs was not affected by scoliosis despite the abnormal spinal overgrowth. Moreover, the peak growth of lower extremities occurred earlier than that of pelvis and spine in both patients with AIS and healthy adolescents.
Bao et al. (17) reported no significant differences in the pelvic height between AIS patients and healthy girls with different maturity statuses, making the HOP a reliable reference for the normalization of LLL and LOS in AIS patients. In the present study, RatioSL and RatioSP were significantly higher in patients with AIS at Risser ≥4 compared to that of healthy controls (P=0.033 and 0.003, respectively), implying that the final spinal length was longer in patients with AIS. Meanwhile, RatioPL showed no significant differences between patients with AIS and healthy girls in both the Risser 0 and Risser ≥4 groups (P=0.499 and 0.303, respectively). The results revealed that the pathogenesis of AIS does not alter the final length of lower limbs. A peri-pubertal study that compared anthropometric measurements between female patients with AIS and controls at different chronological ages also suggested that the final length of the lower limbs is similar between AIS and healthy controls (15). Our study provided further evidence in support of this.
The onset and progress of pubertal growth varied significantly between individuals, even at a similar age. In order to avoid inaccurate evaluation of growth using age, skeleton maturity was confirmed in this study. Although the menarche is a landmark during the pubertal growth of girls, it could not assess growth potential reliably and accurately. Song et al. (18) proposed that the closure of TC is related to the PHV closely. Nault et al. (19) further reported that Risser 0 with the closed TC implied a curve acceleration phase. Therefore, we divided all subjects into 3 groups based on the status of TC, and Risser sign: the pre-PHV group, the during-PHV group, the post-PHV group. Dimeglio et al. (10) elucidated that the peak growth is a combination of three micro-peaks during puberty in healthy adolescents and that the peak growth of the spine and pelvis are later than that of lower extremities. Another study (20,21) by the same team based on anthropometric measurements showed the relative velocity between sitting height and the lower limbs during puberty in adolescents, and the results have further suggested a similar conclusion. Cheung et al. (15) also suggested in an anthropometric study that the peak growth of the lower extremities occurred earlier than that of the spine in both patients with AIS and healthy girls. Our quantified results using full-body radiographic measurements also confirmed these previous conclusions. The change in ratios from the pre-PHV to the post-PHV illustrated the similar trends between patients with AIS and healthy adolescents: RatioSL and RatioPL were significantly lower in the during-PHV group compared to the other two groups (all P<0.02), indicating that the PHV of the lower limbs is earlier than the PHVs of spine and pelvis. Nault et al. (19) found that Risser 0 with the closed TC was the best predictor of the beginning of the curve acceleration phase, which was further confirmed by Shi et al. (22). Regarding the current study, subjects in the during-PHV group were with a closed TC and Risser 0, suggesting that they were at the beginning of the curve acceleration phase and that the peak growth of the lower limbs has just passed. Therefore, RatioSL and RatioPL were significantly lower in this group.
It has been reported that delayed menarche and PHV in AIS patients can lead to a delayed and prolonged period of rapid growth (23,24). The longer spinal length in AIS girls thus may be due to a lengthened period of growth. However, the peak growth velocity of lower limbs emerges before the beginning of the curve acceleration phase. As a result, the final length of the lower limbs may not be affected by the prolonged period of growth, which also indicated that the sequence of reaching PHV for lower limbs and spine in AIS patients is consistent with healthy subjects. In addition, a new consensus was recently reached that the anterior spinal overgrowth was caused mainly by the altered loading pattern in AIS patients instead of a primary growth disturbance (8,9). Nevertheless, the lower extremities and pelvis were not subjected to changed loading, indicating that the growth of the lower extremities and pelvis are not affected in AIS patients. RatioSP showed no significant differences between the three maturity subgroups in the control group (P=0.884). In contrast, RatioSP was higher in the post-PHV AIS group (P=0.003), showing only abnormal spinal overgrowth in AIS.
Several limitations of the present study need to be addressed. First, this study was limited by the relatively small sample size confined to girls with the single thoracic curve. Only patients with thoracic curve were recruited in the cause of allowing consistency. The lumbar curve is less prevalent than the thoracic curve. As for lumbar AIS, the axial vertebral rotation in the lumbar curve is unavoidable, as is sagittal thoracolumbar kyphosis, both of which would affect the actual length of the spine (25). Nevertheless, the conclusion of this investigation may still apply to all different kinds of AIS, including lumbar scoliosis. These findings were based on a single-center study and should be validated in different centers or ethnicities. Besides, in order to further illuminate the growth pattern of the lower extremities in the progression of AIS, a longitudinal investigation is currently underway.
Conclusions
This is the first study to specifically quantify the growth pattern of the lower extremities in AIS patients with whole-body radiographic images. Our results revealed that the final length of the lower extremities was not affected by scoliosis, despite the abnormal spinal overgrowth and that the fact that the peak growth of the lower extremities is earlier than that of pelvis and spine in both patients with AIS and healthy adolescents. These results suggested that the lower limb growth pattern was not altered in AIS patients.
Acknowledgments
We want to thank all participating subjects, and MS Zhang Linlin for radiograph collecting.
Funding: The current study was funded by the Natural Science Foundation of Youth Fund Projects of Jiangsu Province (grant number BK20180122); and supported by Jiangsu Provincial Key Medical Center (grant number YXZXA2016009).This work was also supported by the Special Funds for Health Science and Technology Development of Nanjing City (No. YKK18092).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee. The ethics committee of our hospital approved this prospective study; the informed consent was obtained from all subjects included in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bai J, Chen K, Wei Q, et al. Selecting the LSTV as the Lower Instrumented Vertebra in the Treatment of Lenke Types 1A and 2A Adolescent Idiopathic Scoliosis. Spine (Phila Pa 1976) 2018;43:E390-8. [Crossref] [PubMed]
- Yu CG, Grant CA, Izatt MT, et al. Change in Lung Volume Following Thoracoscopic Anterior Spinal Fusion Surgery. Spine (Phila Pa 1976) 2017;42:909-16. [Crossref] [PubMed]
- Negrini S, Minozzi S, Bettany-Saltikov J, et al. Braces for idiopathic scoliosis in adolescents. Spine (Phila Pa 1976) 2016;41:1813-25. [Crossref] [PubMed]
- Shi L, Wang D, Hui SCN, et al. Volumetric changes in cerebellar regions in adolescent idiopathic scoliosis compared with healthy controls. Spine J 2013;13:1904-11. [Crossref] [PubMed]
- Liu Z, Zhu Z, Guo J, et al. Analysis of body growth parameters in girls with adolescent idiopathic scoliosis: Single thoracic idiopathic scoliosis versus single lumbar idiopathic scoliosis. Stud Health Technol Inform 2012;176:195-201. [PubMed]
- Yu HG, Zhang HQ, Zhou ZH, et al. High ghrelin level predicts the curve progression of adolescent idiopathic scoliosis girls. Biomed Res Int 2018;2018:9784083. [Crossref] [PubMed]
- Schlösser TPC, Van Stralen M, Chu WCW, et al. Anterior overgrowth in primary curves, compensatory curves and junctional segments in adolescent idiopathic scoliosis. PLoS One 2016;11:e0160267. [Crossref] [PubMed]
- Brink RC, Schlösser TPC, Colo D, et al. Anterior Spinal Overgrowth Is the Result of the Scoliotic Mechanism and Is Located in the Disc. Spine (Phila Pa 1976) 2017;42:818-22. [Crossref] [PubMed]
- Bao H, Liu Z, Bao M, et al. Predicted final spinal height in patients with adolescent idiopathic scoliosis can be achieved by surgery regardless of maturity status. Bone Joint J 2018;100-B:1372-6. [Crossref] [PubMed]
- Dimeglio A. Growth in pediatric orthopaedics. J Pediatr Orthop 2001;21:549-55. [Crossref] [PubMed]
- Melhem E, Assi A, El Rachkidi R, et al. EOS®biplanar X-ray imaging: concept, developments, benefits, and limitations. J Child Orthop 2016;10:1-14. [Crossref] [PubMed]
- Nash CL, Moe JH. A study of vertebral rotation. J Bone Joint Surg Am 1969;51:223-9. [Crossref] [PubMed]
- Hasegawa K, Okamoto M, Hatsushikano S, et al. Standing sagittal alignment of the whole axial skeleton with reference to the gravity line in humans. J Anat 2017;230:619-30. [Crossref] [PubMed]
- Urbaniak JR, Schaefer WW, Stelling FH. Iliac apophyses. Prognostic value in idiopathic schliosis. Clin Orthop Relat Res 1976.80-5. [PubMed]
- Siu King Cheung C, Tak Keung Lee W, Kit Tse Y, et al. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: A study of 598 patients. Spine (Phila Pa 1976) 2003;28:2152-7. [Crossref] [PubMed]
- Yim APY, Yeung HY, Hung VWY, et al. Abnormal Skeletal Growth Patterns in Adolescent Idiopathic Scoliosis—A Longitudinal Study Until Skeletal Maturity. Spine (Phila Pa 1976) 2012;37:E1148-54. [Crossref] [PubMed]
- Bao H, Liu Z, Yan P, et al. Disproportionate growth between the spine and pelvis in patients with thoracic adolescent scoliosis: A new look into the pattern’s growth. Bone Joint J 2015;97-B:1668-74. [Crossref] [PubMed]
- Song KM, Little DG. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. J Pediatr Orthop 2000;20:286-8. [Crossref] [PubMed]
- Nault ML, Parent S, Phan P, et al. A modified risser grading system predicts the curve acceleration phase of female adolescent idiopathic scoliosis. J Bone Joint Surg Am 2010;92:1073-81. [Crossref] [PubMed]
- Dimeglio A, Canavese F. Progression or not progression? How to deal with adolescent idiopathic scoliosis during puberty. J Child Orthop 2013;7:43-9. [Crossref] [PubMed]
- DiMeglio A, Canavese F, Charles P. Growth and adolescent idiopathic scoliosis: When and how much? J Pediatr Orthop 2011;31:S28-36. [Crossref] [PubMed]
- Shi B, Mao S, Xu L, et al. Integrated Multidimensional Maturity Assessments Predicting the High-risk Occurrence of Peak Angle Velocity during Puberty in Progressive Female Idiopathic Scoliosis. Clin Spine Surg 2017;30:E491-6. [Crossref] [PubMed]
- Mao SH, Jiang J, Sun X, et al. Timing of menarche in Chinese girls with and without adolescent idiopathic scoliosis: Current results and review of the literature. Eur Spine J 2011;20:260-5. [Crossref] [PubMed]
- Chazono M, Soshi S, Kida Y, et al. Height velocity curves in female patients with idiopathic scoliosis. Stud Health Technol Inform 2012;176:202-5. [PubMed]
- Seki S, Kawaguchi Y, Nakano M, et al. Rod rotation and differential rod contouring followed by direct vertebral rotation for treatment of adolescent idiopathic scoliosis: Effect on thoracic and thoracolumbar or lumbar curves assessed with intraoperative computed tomography. Spine J 2016;16:365-71. [Crossref] [PubMed]


