Heart transplantation in 47 children: single-center experience from China
Introduction
The first pediatric heart transplantation was carried out by Adrian Kantrowitz in 1967, nevertheless, the patient died within several hours after operation (1). Hereafter, the number and survival rate of pediatric heart transplantation have increased gradually, thanks to the advance of surgical techniques, organ protection, immunosuppressant, and perioperative management. In recent years, approximately 600 cases of pediatric transplantation were carried out each year, mainly in Europe and North America. Up till Jun 30th, 2016, the number of pediatric heart transplants accumulated to 13,943 cases globally. In China, heart transplantation in children started in 1994 (2), with just over a hundred cases up to now, far from meeting the needs of treating end-stage heart disease in children. This article retrospectively analyzed 47 pediatric heart transplantations in a single-center, regarding the clinical features of the donors and recipients, perioperative information, postoperative complications, and short- and mid-term survival rate.
Methods
All donor grafts were donated to the Red Cross Society of Hubei Province and allocated by the China Organ Transplant Response System. The study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (IORG No. IORG0003571) and performed in accordance with the national program for deceased organ donation in China (national protocol for China category I). The clinical and research activities are consistent with the principles of the declaration of Istanbul and declaration of Helsinki. Written informed consent was obtained from all patients or their guardians.
Study population
We retrospectively analyzed the heart transplantations in patients under 18 years old performed at our institution between Sep 1st, 2008 and Dec 31st, 2018. The study collected the clinical features of recipients, information of donors, surgical parameters, and postoperative survival rate and complications. Continuous variables were presented as mean ± standard deviation or median, and categorical variables are presented as percentage. The primary end-point was all-cause mortality or need for re-transplantation. The deadline for follow-up was June 30th, 2019.
Organ preservation and operation technique
A uniform method of preservation was applied to all donor hearts and consisted of 1 L of cold (4 °C) histidine-tryptophan-ketoglutarate (HTK) solution during transport. Additionally, 500 mL of HTK solution was perfused before implantation, and a typical biatrial or bicaval procedure with moderate hypothermia (32 °C) was performed. The need for post-transplant mechanical [intra-aortic balloon pump (IABP) or extra-corporeal membrane oxygenation (ECMO)] or inotropic support was determined by the surgeon depending on intraoperative transesophageal echocardiography (TEE), visualization of the heart and hemodynamic monitoring.
Immunosuppressive therapy
Interleukin-2 receptor antagonist (IL-2RA, basiliximab) and methylprednisolone were used for induction therapy. The first dose of basiliximab (20 mg for children <35 kg, 40 mg for children ≥35 kg) was administered intravenously 2 h prior to the operation. The second dose was administered intravenously 4 days after transplantation. For normally developed children over 12 years old, the dose of methylprednisolone is similar to that of adults. For children less than 12 years old, first dose of methylprednisolone (10 mg/kg) was added into the priming solution of the cardiopulmonary bypass (CPB) machine, and second dose (10 mg/kg) was administered intravenously before aortic cross-clamp removal. Upon return to the ICU, patients were administered 2 mg/kg of methylprednisolone intravenously every 8 h, and the dose gradually decreased. Three days later, prednisone (1 mg/kg) was orally taken twice a day. The dose of prednisone was gradually reduced to the maintenance dose 0.2 mg/kg/day. Maintenance immunotherapy consisted of tacrolimus (0–90 days, 10 ng/mL; 90 days–1 year, 8–10 ng/mL; >1 year, 5–8 ng/mL), mycophenolate (600 mg/m2/day) and prednisone were used for all recipients.
Results
Clinical characteristics
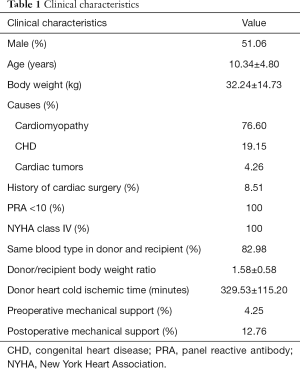
The patients aged from 3 months to 18 years, with a mean age of 10.34±4.80 years. There were 24 males and 23 females. The average body weight was 32.24±14.73 kg. The mean pulmonary artery pressure was 32.88±11.83 mmHg before operation. Preoperative diagnosis included 36 cases (76.60%) of cardiomyopathy, 9 cases (19.15%) of complex congenital heart disease (CHD), and 2 cases (4.26%) of cardiac tumor; 4 patients (8.51%) received cardiac surgery before. All patients were assessed as New York Heart Association (NYHA) class IV heart failure. All patients had a PRA level of lower than 10%; 39 cases (82.98%) received donor hearts with the same ABO blood type, 8 received compatible donor hearts (17.02%). The mean donor/recipient body weight ratio was 1.58±0.58. The cold ischemic time was 329.53±115.20 minutes on average (median 370.00 minutes), including 24 cases (51.06%) longer than 6 hours (Table 1).
Full table
Surgical information
Aortic clamping time ranged from 20–105 minutes (average 35.36±16.37 minutes). The duration of intraoperative CPB time averaged 119.00±53.47 minutes (range, 71–217 minutes). Thirty-seven cases used bicaval technique, and 10 cases used biatrial technique. In order to promote long-term growth and development, we used prolene sutures in all posterior anastomosis and continuous suture with PDS sutures in all anterior anastomosis. There were 3 cases of dextrocardia, 2 cases combined with correction of anomalous pulmonary venous connection, 2 cases combined with correction of aortic coarctation, and 1 case combined with occlusion of major aorto-pulmonary collateral. Delayed sternal closure was used in 6 patients.
Perioperative mechanical circulatory support
There were 2 patients using ECMO preoperatively, both of whom were bridged to transplant successfully. In 6 cases, mechanical assistance was used postoperatively due to the low cardiac output, 4 of which were ECMO only and 2 were IABP combined with ECMO. The wean-off rate of ECMO was 100%.
Incidence of complications and survival rate
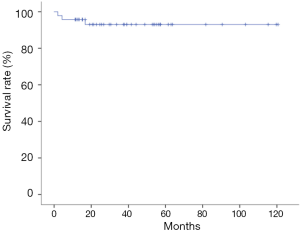
The average duration of postoperative mechanical ventilation was 32.00 (18.50–54.00) hours, and the average ICU stay was 7.00 (4.94–11.28) days. Postoperative complications occurred in 20 cases (42.55%), including 7 cases (14.89%) of low cardiac output, 5 cases (10.63%) of arrhythmia, 7 cases (14.89%) of renal insufficiency, 3 cases (6.38%) of acute rejection, and 1 case (2.13%) of hematological complication. At 3 weeks after operation, the average left ventricular ejection fraction (LVEF) of the patients was 67.30%±5.13%. Forty-six patients discharged after rehabilitation. One patient died from pneumonia on the postoperative day 59. The mean follow-up time was 41.80±30.51 months. The 1-year, 3-year, and 5-year survival rate after operation was 95.74%, 93.01%, and 93.01% respectively (Figure 1).
Discussion
Heart transplantation, an experimental, high-risk technique as it used to be, through the over 50 years’ development, has already become an effective approach for treating adult and pediatric end-stage heart failure. However, owing to the restricted donor source, there was no obvious increase in the scale of pediatric heart transplantation in the last decade.
In China, heart transplantation in adults has been developed rapidly, while heart transplantation in children still lags, with only 127 cases have been completed nationwide by Dec 31st, 2017. Some difficulties of pediatric heart transplantation might account for the slow development. In the first place, it’s hard to find matched donors, especially for infant recipients. Secondly, pediatric heart anomalies are usually complicated, accompanied by anomalies in aorta or pulmonary vasculature. Concurrent correction procedures add to the technical difficulty. Other obstacles include the surgical history, either palliative or corrective in some patients with complex CHD, special perioperative management in infant patients, and need of adjustment in anti-rejection regimen due to the body growth of children. In this article, we retrospectively analyzed 47 cases of pediatric heart transplantation in our hospital, hoping to provide some reference for the development of pediatric heart transplantation in our country.
In the U.S., there has been no universal indication for heart transplantation in children yet. Each center has their own criteria. Stage D heart failure that needs continuous intravenous inotropic agents, mechanical ventilation or mechanical circulatory support, and is unmanageable through routine operation, should go for heart transplantation. Stage C heart failure could be considered as an indication for heart transplantation given the potential risk of sudden death and the persistent elevation of pulmonary vascular resistance (3). In fact, indications for pediatric heart transplantation have been changing over time. In the 1980s, heart transplantation was superior to Norwood procedure in terms of survival rate, and hypoplastic left heart syndrome (HLHS) was the major indication for pediatric heart transplantation (4,5). However, with the improving success rate and lack of infant donors for transplantation, Norwood procedure gradually dominated HLHS management, leaving cardiomyopathy to be the major cause for pediatric heart transplantation. Another reason accounting for the changing status of transplantation is the better prognosis of palliative surgery in patients with complex CHD, enabling them to postpone heart transplantation until adulthood. So far, the main cause of heart transplantation is complex CHD in patients under 1-year-old, and cardiomyopathy in patients over 1-year-old. The 10-year survival rate of complex CHD after transplantation is lower than that of cardiomyopathy, for approximately 10%. The main difference lies in the short-term survival after operation (6). The long-term survival rate of complex CHD is significantly higher than that of cardiomyopathy after the first year post-transplantation (median survival time 22 vs. 16.5 years) (7). In our center, the main cause of pediatric heart transplantation is cardiomyopathy; 59.57% of the recipients are between the ages of 10 and 17 years. Infant complex CHD makes up only a minor portion of heart transplantation. The two diseases show no significant difference in survival after transplantation, which might be owing to the small sample size and short follow-up time.
According to statistical data, around 20–25% of the children with end-stage heart disease die before they find a suitable donor. How to improve the utilization of donor hearts is the pending issue in pediatric heart transplantation. Cold ischemic time is the key factor affecting the quality of donor hearts. Cold ischemic time is not necessarily associated with postoperative complications, but a significantly lower survival rate is observed when cold ischemic time is longer than 4 hours. Interestingly, cold ischemic time of more than 4 hours does not significantly change the survival rate of transplantations in complex CHD. The international level of cold ischemic time is 3.5–3.7 hours on average, which is hard to achieve in China owing to the efficiency of public transportation like civil aviation and high-speed rail. The myocardial protection strategy used in our center, composing of rapid induction of cardiac arrest with modified St. Thomas cardioplegic solution, perfusion with Histidine-Ketoglutarate-Tryptophan (HTK) solution, preservation with stratified ice crystals, and reperfusion with HTK solution after donor heart trim, could effectively prolong the safety time limit of cold ischemia. We retrospectively analyzed the 122 cases of heart transplantation with long cold ischemic time (6 to 8 hours) in our center from Jan 1st, 2015 and Dec 31st, 2017. The average cold ischemic time was 393.5 minutes. The usage rate of IABP was 36.63%, and that of ECMO was 3.00%. The short term 1-year and 2-year survival rate was 87.1% and 85.1% respectively, which has no significant difference with that of standard donor heart (8). Some literature reported that prolonged cold ischemic time seemed to have no influence on the survival rate of young donor hearts (9). Still, studies with larger sample sizes and longer follow-up time are required for further evaluation of the effect of long cold ischemic time on the survival of pediatric heart transplantation. When choosing and assessing donors, we should take into account factors including age, time from disease onset to organ harvest, dose of vasoactive agents, preoperative organ protection, and recipient’s pulmonary vascular pressure.
For pediatric recipients with poor heart function, unstable hemodynamic, or rare HLA types, mechanical assistance is considered as an effective bridge-to-transplant approach and powerful postoperative support. Ventricular assist devices (VAD) is less applied in heart transplantations in infants or young children because of body weight limitation. So far, only a few types of VAD (like Berlin Heart EXCOR) are appropriate for small children (10,11). ECMO still serves as the major bridge-to-transplant approach and postoperative circulatory support approach. According to the United Network of Organ Sharing (UNOS), in 2,777 cases of pediatric heart transplantation from 2005 to 2012, 617 cases required bridge-to-transplant mechanical assistance, including 428 cases using VAD and 189 cases using ECMO. Compared with the VAD group, patients in the ECMO group were younger and of lower body weight. The short-term (4-month) survival rate in the ECMO group was lower than the direct transplantation group, while the 1-year, 3-year, and 5-year survival rate showed no significant difference between the two groups (12). According to the Scientific Registry of Transplant Recipients (SRTR) and the Public Health Information System (PHIS), ECMO was used in 7.9% of all heart transplantations in children; 47.3% of those who were assisted with ECMO after transplantation were under 1 year of age. The risk factors for postoperative ECMO assistance include age of less than 1 year, age of 1 to 5 years, and bridge-to-transplant with ECMO (13). However, Vanderlaan et al. reported that ECMO assistance, either preoperative or postoperative, would increase the risk of graft failure (14). Longer ECMO assistance was associated with higher in-hospital mortality, while the long-term survival rate after discharge was not affected (13). Another retrospective single-center study showed that ECMO could provide effective hemodynamic support, and improve postoperative survival (15). In our center, satisfactory results were observed in ECMO assisted transplantation, either as bridge-to-transplant or postoperative support. ECMO could help with cardiac insufficiency and reduce the burden of the donor heart, so as to be better adapted to the hemodynamics in the recipient. The key to successful ECMO assistance lies in the choice of recipient and the time-to-support. Nevertheless, ECMO-associated complications and organ injuries before catheterization might hamper the effectiveness of ECMO assistance. This study is limited by the small sample size. Future multi-center researches with larger sample sizes will help to clarify the influence of ECMO assistance on transplantation survival.
One of the difficulties of pediatric heart transplantation with end-stage complex CHD is the complexity of the anomalies. For operating on these patients, sufficient preoperative assessment, deep understanding of the anatomy, and rich surgical experience on complex CHD are required. We innovatively designed several procedures according to the vena cava and pulmonary venous return, and the morphology of atria, pulmonary arteries, and aorta. (I) Transplantation for congenital dextrocardia. Our pioneering technique involves a 90-degree clockwise rotation around the left atrium (16). This results in anastomosis of the left superior pulmonary vein of the donor heart and the left inferior pulmonary vein of the recipient. Then the aorta, inferior and superior vena cava and pulmonary artery were anastomosed continuously. Prosthetic conduits were used if necessary. With this procedure, the CPB time can be reduced by 20%. (II) Hybrid transplantation. End-stage cyanotic complex CHD is usually accompanied by major aorto-pulmonary collaterals. In these patients, interventional occlusion combined with heart transplantation is simpler and brings less injury to the lungs (17). (III) Correction of extra-cardiac anomalies combined with transplantation. For end-stage heart disease with total anomalous pulmonary venous connection (TAPVC), we combined surgical correction of TAPVC with heart transplantation. For end-stage heart disease with aortic coarctation, we combined aortic arch reconstruction using artificial vascular patch with transplantation.
Challenges as it’s facing, pediatric heart transplantation is still a promising treatment for children end-stage heart disease. We retrospectively analyzed 47 cases of pediatric heart transplantation in our center, probably the largest sample size in China. The short- and mid-term survival in this study is comparable to the level reported by the International Society of Heart and Lung Transplantation (ISHLT), which is quite satisfactory. Through our practice, we established a more complete procedure for pediatric heart transplantation, which would hopefully promote this technique in China.
Acknowledgements
This project is attributed to the Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. We are indebted to Dr. Kailun Zhang, Shiliang Xiao, Jiahong Xia and Xinling Du for their generous assistance.
Funding: This work was supported by National Key Research and Development Program (No. 2016YFA0101100).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.99). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol for the research project has been approved by Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (IORG No. IORG0003571) and it conforms to the provisions of the Helsinki Declaration as revised in 2013. Written informed consent was obtained from all patients or their guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kantrowitz A, Haller JD, Joos H, et al. Transplantation of the heart in an infant and an adult. Am J Cardiol 1968;22:782-90. [Crossref] [PubMed]
- Sun P, Zhang X, Cheng G, et al. Heart Transplantation in Infant-a report of 1 case. Acad J Sums 2001;22:261-3.
- Canter CE, Shaddy RE, Bernstein D, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;115:658-76. [Crossref] [PubMed]
- Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol 1980;45:87-91. [Crossref] [PubMed]
- Boucek MM, Mathis CM, Razzouk A, et al. Indications and contraindications for heart transplantation in infancy. J Heart Lung Transplant 1993;12:S154-8. [PubMed]
- Dipchand AI, Kirk R, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Heart Transplantation Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:979-88. [Crossref] [PubMed]
- Alsaied T, Khan MS, Rizwan R, et al. Pediatric Heart Transplantation Long-Term Survival in Different Age and Diagnostic Groups: Analysis of a National Database. World J Pediatr Congenit Heart Surg 2017;8:337-45. [Crossref] [PubMed]
- Shafiq F, Wang Y, Li G, et al. Clinical outcome of donor heart with prolonged cold ischemic time: A single-center study. J Card Surg 2020;35:397-404. [Crossref] [PubMed]
- Park CS, Villa CR, Lorts A, et al. Is there an optimal organ acceptance rate for pediatric heart transplantation: "A sweet spot"? Pediatr Transplant 2018;22:e13149. [Crossref] [PubMed]
- Writing Committee Members, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [PubMed]
- Weinstein S, Bello R, Pizarro C, et al. The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg 2014;147:697-704; discussion 704-5. [Crossref] [PubMed]
- Wehman B, Stafford KA, Bittle GJ, et al. Modern Outcomes of Mechanical Circulatory Support as a Bridge to Pediatric Heart Transplantation. Ann Thorac Surg 2016;101:2321-7. [Crossref] [PubMed]
- Godown J, Bearl DW, Thurm C, et al. Extracorporeal membrane oxygenation use in the first 24 hours following pediatric heart transplantation: Incidence, risk factors, and outcomes. Pediatr Transplant 2019;23:e13414. [Crossref] [PubMed]
- Vanderlaan RD, Manlhiot C, Conway J, et al. Perioperative factors associated with in-hospital mortality or retransplantation in pediatric heart transplant recipients. J Thorac Cardiovasc Surg 2014;148:282-9. [Crossref] [PubMed]
- Su JA, Kelly RB, Grogan T, et al. Extracorporeal membrane oxygenation support after pediatric orthotopic heart transplantation. Pediatr Transplant 2015;19:68-75. [Crossref] [PubMed]
- Dong N, Liu J, Wang G, et al. Three cases of Orthotopic Cardiac Transplantation for Congenital Dextrocardia. Chin J Organ Transplant 2019;40:111-5.
- Shang X, Lu R, Dong N, et al. Heart Transplantation combined with Interventional Therapy for Complex Congenital Heart Disease. Chin J Intervent Cardiol 2017;25:227-8.