
A therapeutic perspective for proliferative vitreoretinopathy based on the inhibition of epithelial-mesenchymal transition by miR-194
An exciting new study by Cui et al. (1) “miR-194 suppresses epithelial-mesenchymal transition of retinal pigment epithelial cells by directly targeting ZEB1” published in Annals of Translational Medicine adds pieces in the framework of the regulation of cellular plasticity of retinal pigment epithelial (RPE), a commitment necessary to maintain a properly functioning and organized retina. Proliferative vitreoretinopathy (PVR) is main cause of failure of surgical treatment of rhegmatogenous retinal detachment (2). PVR is characterized by epithelial-mesenchymal transition (EMT) and hypertrophy of RPE. Clinical and experimental evidence has shown that RPE cells undergo EMT to adopt a fibroblastic phenotype, indicating that intact cell-cell adhesions and functioning signaling pathways such as Wnt and Hippo signaling, as well as EMT proteins, are essential for the maintenance of the RPE phenotype (3).
MiR-194 had previously been found expressed in the epithelia of organ sensor, the inner ear membrane and the retina of developing mice (4,5). The work of Cui et al. (1) confirms the abundance of miR-194 in the rat retina and human ARPE-19 cells and provides an overview of the role of miR-194 in the EMT of ARPE-19 cells. Notably, miR-194 overexpression is shown for the first time to suppress effectively EMT of RPE by targeting the EMT regulator zinc finger E-Box binding homeobox 1 (ZEB1). Cui et al. (1) develop in vitro and in vivo experiments coming to conclusion that miR-194 is a potential therapeutic tool in PVR. Profiling of mRNA expressions in RPE cells overexpressing miR-194 shows enrichment for genes involved in infection, inflammation, Hippo pathway, NF-κB pathway, and for pathways closely related to RPE functions as phagocytosis, cell adhesion and interaction with extra-cellular matrix (1). Furthermore, miR-194 overexpression suppresses proliferation and migration of RPE cells. Overall, miR-194 promises to act as an EMT modulator and on specialized functions of RPE.
The molecular and cellular mechanisms underlying an EMT can be initiated by multiple extracellular signals depending on the physiological or pathological context (6-9). In RPE cells, various signals induce EMT (10-14). The proven connection between miR-194 and ZEB1 in RPE cells by Cui et al. (1) represents a milestone for a better understanding of EMT. ZEB1 is believed an essential driver of cellular plasticity and consequently of progression from EMT activation and tumorigenesis to advanced metastases (15,16). In addition to the mutually stimulating and coherent loop TGFβ-ZEB1, ZEB1 transcription in RPE cells undergoing EMT could be mediated by Hippo pathway activation (17). ZEB1 promotes EMT by repressing stemness-inhibiting microRNAs including miR-200 family, miR-203, miR-183 and miR-141 (18). By means of these mechanisms, ZEB1 links the activation of EMT and the maintenance of mobile cells.
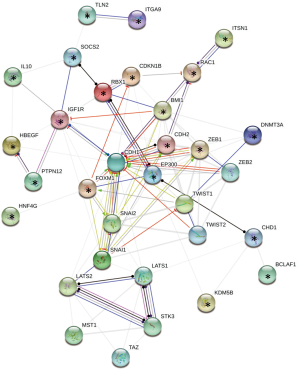
Transforming growth factor-β proteins (TGF-β) oversee and direct many aspects of cell development, differentiation, and homeostasis (19,20). miR-194 overexpression suppresses TGF-β1-induced EMT and restricts ZEB1 mRNA and protein levels in ARPE-19 cells. Thus, Cui et al. (1) hypothesize and confirm that ZEB1 is a direct target of miR-194. To further support the importance of ZEB1 inhibition by miR-194 in RPE’s EMT, ZEB1 silencing also attenuates TGF-β1-induced EMT and promotes cell growth arrest, independently from classical TGF-β1 pathway. Interestingly, ZEB1-regulated genes in RPE cells show changes that counteract what has been observed in other cell types, implying context-dependent regulation of the ZEB1 pathway (1). We represent a possible scenario based on experimentally validated target genes of miR-194-5p. Figure 1 shows the interaction network among proteins controlled by hsa-miR-194-5p and others involved in EMT and Hippo pathways. ZEB1 cross-talks with typical EMT proteins and others as BMI1, EP300, IGFR1R and FOXM1, which connect with Hippo pathway mainly via SNAI1 and SNAI2. In summary, miR-194 can modulate EMT and Hippo pathways through different routes although ZEB1 remains central for this outcome.
On a more practical level, the work of Cui et al. (1) demonstrates effectiveness of miR-194 manipulation in RPE cells. A rat PVR model was created by vitreous injection of platelet-rich plasma added with human ARPE-19 cells combined to miR-194 or miR-194-inhibitor. In line with expectations, miR-194 supplied exogenously attenuates ZEB1 immunostaining and EMT of RPE cells in the rat retina. This discovery brings together two new potential targets for the PVR care. In the future, it would be important to investigate the context dependency of miR-194 action, identifying proteins cooperating with miR-194 and its target genes other than ZEB1 yet to be ascertained.
MicroRNAs are pleiotropic agents each of which potentially acts on many target genes and diverse cellular functions. The overall profile of microRNAs expressed in a cell context integrates signals and modulate regulatory circuitries, cooperating with transcription factors in dynamically establishing mRNA levels (23,24). Future researchers should aim at improving understanding the role of ZEB1 and miR-194 in RPE and testing effectiveness of miR-194 modulation alone and in combination with other agents. Elevating or inhibiting the level of microRNAs targeting known genes and signaling pathways could reveal strategic for restoring homeostasis and functions in the cell. Precisely because they are supported by multiple targeting capability, certain microRNAs could be capable to subvert the hierarchical relationships among diverse regulation levels in certain pathological contexts.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.181). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cui L, Lyu Y, Jin X, et al. miR-194 suppresses epithelial-mesenchymal transition of retinal pigment epithelial cells by directly targeting ZEB1. Ann Transl Med 2019;7:751. [Crossref] [PubMed]
- Kwon OW, Song JH, Roh MI. Retinal Detachment and Proliferative Vitreoretinopathy. Dev Ophthalmol 2016;55:154-62. [Crossref] [PubMed]
- Chen Z, Shao Y, Li X. The roles of signaling pathways in epithelial-to-mesenchymal transition of PVR. Mol Vis 2015;21:706-10. [PubMed]
- Du J, Zhang X, Cao H, et al. MiR-194 is involved in morphogenesis of spiral ganglion neurons in inner ear by rearranging actin cytoskeleton via targeting RhoB. Int J Dev Neurosci 2017;63:16-26. [Crossref] [PubMed]
- Xu S, Witmer PD, Lumayag S, et al. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem 2007;282:25053-66. [Crossref] [PubMed]
- Bifari F, Decimo I, Pino A, et al. Neurogenic Radial Glia-like Cells in Meninges Migrate and Differentiate into Functionally Integrated Neurons in the Neonatal Cortex. Cell Stem Cell 2017;20:360-373.e7. [Crossref] [PubMed]
- Ricciardi M, Malpeli G, Bifari F, et al. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS One 2012;7:e35639. [Crossref] [PubMed]
- Ricciardi M, Zanotto M, Malpeli G, et al. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer 2015;112:1067-75. [Crossref] [PubMed]
- Yang S, Li H, Li M, et al. Mechanisms of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Discov Med 2015;20:207-17. [PubMed]
- Chen Z, Mei Y, Lei H, et al. LYTAK1, a TAK1 inhibitor, suppresses proliferation and epithelialmesenchymal transition in retinal pigment epithelium cells. Mol Med Rep 2016;14:145-50. [Crossref] [PubMed]
- Feng H, Zhao X, Guo Q, et al. Autophagy resists EMT process to maintain retinal pigment epithelium homeostasis. Int J Biol Sci 2019;15:507-21. [Crossref] [PubMed]
- Miao Q, Xu Y, Yin H, et al. KRT8 phosphorylation regulates the epithelial-mesenchymal transition in retinal pigment epithelial cells through autophagy modulation. J Cell Mol Med 2020;24:3217-28. [Crossref] [PubMed]
- Nagasaka Y, Kaneko H, Ye F, et al. Role of Caveolin-1 for Blocking the Epithelial-Mesenchymal Transition in Proliferative Vitreoretinopathy. Invest Ophthalmol Vis Sci 2017;58:221-9. [Crossref] [PubMed]
- Zhang J, Yuan G, Dong M, et al. Notch signaling modulates proliferative vitreoretinopathy via regulating retinal pigment epithelial-to-mesenchymal transition. Histochem Cell Biol 2017;147:367-75. [Crossref] [PubMed]
- Gregory PA, Bracken CP, Smith E, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell 2011;22:1686-98. [Crossref] [PubMed]
- Postigo AA, Depp JL, Taylor JJ, et al. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J 2003;22:2453-62. [Crossref] [PubMed]
- Liu Y, Xin Y, Ye F, et al. Taz-tead1 links cell-cell contact to zeb1 expression, proliferation, and dedifferentiation in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2010;51:3372-8. [Crossref] [PubMed]
- Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 2009;11:1487-95. [Crossref] [PubMed]
- Hao Y, Baker D, Ten Dijke P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int J Mol Sci 2019. [Crossref] [PubMed]
- Palomares-Ordóñez JL, Sanchez-Ramos JA, Ramirez-Estudillo JA, et al. Correlation of transforming growth factor beta-1 vitreous levels with clinical severity of proliferative vitreoretinopathy in patients with rhegmatogenous retinal detachment. Arch Soc Esp Oftalmol 2019;94:12-7. [PubMed]
- Chou CH, Shrestha S, Yang CD, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res 2018;46:D296-302. [Crossref] [PubMed]
- Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607-13. [Crossref] [PubMed]
- Kaneko H, Terasaki H. Biological Involvement of MicroRNAs in Proliferative Vitreoretinopathy. Transl Vis Sci Technol 2017;6:5. [Crossref] [PubMed]
- Malpeli G, Barbi S, Tosadori G, et al. MYC-related microRNAs signatures in non-Hodgkin B-cell lymphomas and their relationships with core cellular pathways. Oncotarget 2018;9:29753-71. [Crossref] [PubMed]


