
Increased the risk of heart failure and comorbidities in patients with gout treatment: a population-based cohort study
Introduction
Because of its unique geographical and racial distribution, the rate of hyperuricemia is high in Taiwan, with a prevalence of 43.7% in men and 27.4% in women (1). Many studies have suggested that hyperuricemia is an independent risk factor for cardiovascular morbidity and mortality (2-4). According to nutritional and health surveys in Taiwan, research, including that on medical history, physical activity, food frequency, and fasting blood parameters, has revealed that the uric acid levels and prevalence of hyperuricemia decreased in 1993–1996 and 2005–2008 but not the prevalence of gout (5). Although the nature of the relationship between gout and cardiovascular disease (CVD) remains unclear due to shared risk factors, such as diabetes, metabolic syndrome, and obesity (6-8), several epidemiologic reports concerning populations in America or Asia have mentioned that gout has an independent role in CVD (9-11). One review article suggested that patients with HF also suffered from hyperuricemia and gout (12). A case-control study showed the occurrence of gouty arthritis in HF patients (13). The Cameroon HF study revealed gout was one of the co-morbidities around 16.4% of HF subjects (14). HF is an important public health problem that affects an estimated 26 million individuals and results in more than 1 million hospitalizations each year. HF also causes an enormous economic burden worldwide (15). The Framingham Offspring Study suggested that hyperuricemia was an independent predictor for HF, and the other cohort also confirmed the relationship between serum uric acid and HF hospitalization in both sexes (16,17). Because of limited studies on the association between gout treatment and HF (7,18,19), we designed the present study to investigate the association between gout treatment and HF.
Methods
Data source
The National Health Insurance (NHI) program was launched in 1995 and has since covered over 99% of the 23.74 million people residing in Taiwan (http://www.nhi.gov.tw/english/index.aspx). We obtained data files of electronic claims from the Longitudinal Health Insurance Database (LHID), which contains the claims data of 1 million people randomly selected from the insured population. The LHID makes available linked data from 1996 to 2011 for every insurant in the NHI program. The identification numbers of all patients and their reimbursement data from the LHID are encrypted to protect their privacy. Diseases in the claims data were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). This study was exempted from a full ethical review by China Medical University and Hospital Research Ethics Committee (IRB permit number: CMUH104-REC2-115-R4).
Sampled participants
Patients 20 years of age and older who were newly diagnosed with gout (ICD-9-CM code 274) between 2000 and 2010 constituted the gout cohort. The index date for the patients was the date of their first medical visit for gout. Patients who were diagnosed with HF (ICD-9-CM code 428) at baseline or who were missing information were excluded. The nongout control cohort consisted of patients randomly selected from the LHID without a history of gout. For each gout case, we randomly selected two control persons through frequency matching based on sex, age (5-year periods), and year of index date. The same exclusion criteria were also applied to the nongout controls.
Outcome
The main outcome was outpatient visits or hospitalization with a new diagnosis of HF during the follow-up period. Both cohorts were followed from the index date until diagnosis with HF, withdrawal from the NHI program, or the end date of December 31, 2011.
Comorbidities and medications
The baseline comorbidities and medications considered in this study were diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), chronic kidney disease (ICD-9-CM 580–589), stroke (ICD-9-CM codes 430–438), chronic obstructive pulmonary disease (ICD-9-CM codes 491, 492, 496), asthma (ICD-9-CM code 493), alcohol-related illness (ICD-9-CM codes 291, 303, 305, 571.0, 571.1, 571.2, 571.3), coronary artery disease (ICD-9-CM codes 410–414), prednisolone, and nonsteroid anti-inflammatory drugs (NSAIDs). We hypothesized that antigout drugs, including allopurinol, benzbromarone, probenecid, sulfinpyrazone, and colchicine, have different effects on HF in patients with gout.
Statistical analysis
We first compared the distributions of age (≤34, 35–49, 50–64, and 65+ years), sex, comorbidities, and medications between patients with and without gout using a chi-square test for the categorical variables and a t-test for the continuous variables. The follow-up person-years were used to estimate the incidence density for each cohort. Univariable and multivariable Cox proportional hazard regression models were used to measure the effect of gout on the risk of developing HF. The multivariable models were simultaneously adjusted for age; sex; and the comorbidities of diabetes, hypertension, hyperlipidemia, chronic kidney disease, stroke, chronic obstructive pulmonary disease, asthma, alcohol-related illness, and coronary artery disease; and the medications of prednisolone and NSAIDs. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox model. Further analysis was performed to assess whether antigout drug treatment played a role in the HF outcomes. The Kaplan–Meier method was used to estimate the cumulative HF incidence curve for the gout and nongout cohorts, and the log-rank test was conducted to determine the difference in these curves. All analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC, USA). A two-tailed P value <0.05 was considered statistically significant.
Results
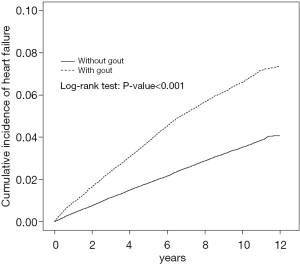
The cohort study included 50,166 patients with gout and 100,332 patients without gout. Table 1 shows the baseline demographic data, comorbidities, and medications of the cohorts. Most patients were ≥50 years of age (51.5%) and men (73.3%). The mean ages of the patients in the gout and nongout cohorts were 51.2 (SD =16.2) and 50.6 (SD =16.4) years, respectively. The patients in the gout cohort were more likely to have comorbidities and medications than those in the nongout cohort (all P values <0.001). During the mean follow-up periods of 7.40 [standard deviation (SD) =3.22] and 7.28 (SD =3.26) years in the gout and nongout cohorts (data not shown), respectively, the cumulative incidence of HF was 3.16% higher among the patients with gout than among those without gout (log-rank P<0.001) by the end of follow-up (Figure 1).
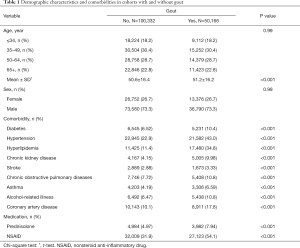
Full table
Overall, the incidence densities of HF were 1.96 times higher in the gout cohort than in the nongout cohort (7.11 vs. 3.63 per 10,000 person-years), with an adjusted HR of 1.57 (95% CI: 1.49–1.67; Table 2). The age-specific incidence of HF increased with age in both cohorts. The age-specific gout cohort to nongout cohort adjusted HR of HF was significantly higher for all age groups. Within both cohorts, a higher incidence of HF occurred among women than among men. The comorbidity-specific adjusted HR of HF indicated that the gout cohort had higher risk than patients in the noungout cohort both without a comorbidity (HR =2.15, 95% CI: 1.85–2.51) and with comorbidity (HR =1.54, 95% CI: 1.28–1.85). In both cohorts, those receiving prednisolone or NSAID treatment displayed a higher incidence of HF than did those without prednisolone or NSAID treatment.
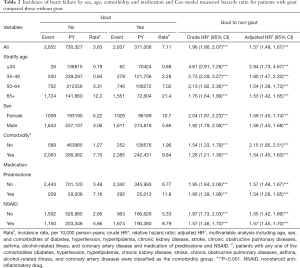
Full table
Table 3 shows the rates of gout and the other risk factors for HF in the study participants. The adjusted HR of developing HF was a 1.06-fold increase (95% CI: 1.06–1.07) with age (every 1 year) and a 1.08-fold increase for women compared with men (95% CI: 1.02–1.14). As expected, patients with diabetes, hypertension, hyperlipidemia, chronic kidney disease, stroke, chronic obstructive pulmonary disease, asthma, alcohol-related illness, and coronary artery disease were more likely to have HF.
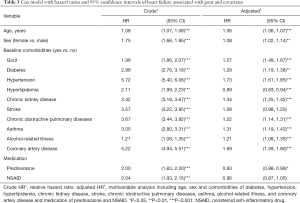
Full table
Table 4 shows the analysis of treatment associated with HF risk among gout patients. Compared with gout patients who did not receive any treatment, gout patients who received any two or more types of anti-gout drugs exhibited a significantly higher risk for HF (adjusted HR =1.64, 95% CI: 1.46–1.85).
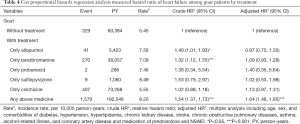
Full table
The risk of HF were 1.82 times higher in the gout cohort than in the nongout cohort among women patients age <50 years (adjusted HR =1.82, 95% CI: 1.15–2.87) (Table 5). The risk of HF were 1.56 times higher in the gout cohort than in the nongout cohort among women patients age >50 years (adjusted HR =1.56, 95% CI: 1.42–1.72).
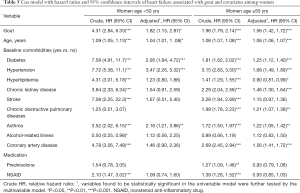
Full table
Table 6 presented the risk of HF among different drug exposure (cumulative exposure dose). Relative to the prednisolone non-users, the risk of HF was significantly associated with decreased risk in higher prednisolone exposure. The results also demonstrated, compared with NSAID non-users, HRs of HF risk was 0.53 (95% CI: 0.35–0.81) for NSAID users with >28,500 mg exposure. The HF risk were still significantly associated with higher risk for ULTs users >1,200 mg compared with ULTs non-users (adjusted HR =1.13, 95% CI: 1.05–1.20) and for ULTs users ≤1,200 mg compared with ULTs non-users (adjusted HR =1.93, 95% CI: 1.63–2.27).
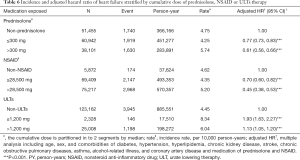
Full table
Discussion
Gout and HF
Outside of Europe and America (20,21), the highest prevalence of gout is in Taiwan (1,22). Our analysis showed the same results as those of previous studies conducted in Taiwan regarding the higher prevalence of gout in men than women (23). A higher HF rate was also noted in our gout group. One Crystal-proven Gout study showed the analysis of relationship between gout and other 8 different CVD. Except transient ischemic attack, significant higher prevalence of other CVD including HF around 14% was noted (24). However, we found that females with gout were more at risk for HF than were males were gout, which is different from other studies that have identified a relationship between high renal excretion of urate and plasma estrogen in women with gout (25,26). In our analysis, the age of women was not the confounder for HF showed in Table 5. In our opinion, different race or gene might be the possible explanation. In the Framingham Offspring Study, gout was associated with poor cardiac pump function, resulting in clinical HF. Myocardial systolic dysfunction was noted in the follow-up analysis and caused elevated mortality in subjects with gout and HF. A higher incidence rate of HF was associated with older age in our study, which confirmed the results of the Quebec study (27). One basic research mentioned gout is also associated with a shorter telomere length which is associated with ageing and therefore with more cardiovascular diseases such as HF (28).
Gout treatment and HF
Prednisolone and NSAIDs
Gout is a low-grade chronic inflammatory condition that promotes thrombogenesis or atherogenesis (22,29). In our analysis for prednisolone, a higher HF rate was found in non-prednisolone user and higher dose of prednisolone could decrease the risk of HF which was consistent with the anti-inflammatory function of prednisolone. In our study, both groups with or without steroid use showed the higher HF risk. Insufficient dosage or poor compliance with steroid use might be the reason for this result (30,31). A relatively high HF rate was noted in those using NSAIDs in our study. Although NSAIDs can inhibit the pathway of cyclooxygenase-2 induced by inflammation and are effective for gout treatment. Unfortunately, in healthy subjects, NSAIDs can have a negative effect on renal function (causing sodium and volume retention), may worsen the condition of renal failure in elderly persons, and can increase HF (12,32).
Colchicine
Colchicine is a potent anti-inflammatory medication extracted from the autumn crocus that has been used for centuries. Its anti-inflammatory mechanism occurs through the inhibition of microtubule generation and tubulin polymerization. Colchicine could inhibit the inflammatory pathway resulting in the stability of native atherosclerotic plaques. The protean effects of colchicines could inhibit neutrophil chemotaxis and activation within a proinflamatory environment causing to reduce the levels of high sensitivity C-reactive protein (33). One randomized clinical trial of colchicine showed a significantly decreased risk of CVD (34), and others have revealed that colchicine can decrease ischemic cardiovascular events (35). In our analysis of colchicine and HF, gout treatment with colchicine was not found to decrease HF. This might be because of the high dose effect or the other mechanisms of colchicine, such as inhibition of spindle formation and cell division, that constrict blood vessels (12).
Xanthine oxidase inhibitor
After myocardial infarction, poor left ventricular remodeling causes the development of HF and myocardial reactive oxygen species (ROS) production, leading to cardiac hypertrophy and dysfunction (36). Xanthine oxidase, a potent enzymatic source of ROS, was demonstrated in HF and shown to promote cardiomyocyte hypertrophy (37). Febuxostat and allopurinol, a xanthine oxidase inhibitor, are usually prescribed for gout treatment. Febuxostat was not analyzed here because during our study period, this type of medicine could not be prescribed in Taiwan. However, one recent publication showed that compared with allopurinol, febuxostat might increase all-cause mortality and cardiovascular mortality (38). Many publications have suggested that long-term use (more than 300 mg/day) of allopurinol reduced cardiovascular hospitalizations and improved cardiovascular mortality (39,40). A study for different dose effect of allopurinol to forearm blood flow showed that higher dose of allopurinol could improve the endothelial function resulting in better myocardial perfusion and improving LV function. This study also mentioned the underlying mechanism for left ventricle remodeling could still be oxidative stress reduction. Different antioxidant effect of allopurinol was dose dependence. Allopurinol also improved endothelial-dependent vasodilatation by 52% compared with probenecid (39). Unfortunately, our results did not indicate improved HF rates. The dose response due to the habit of prescription or the adherence of patients is a possible reason.
Uricosuric drugs
These types of medicines, which include benzbromarone, probenecid, and sulfinpyrazone, block renal tubular urate reabsorption at the proximal renal tubule (41). One study showed that compared with allopurinol, probenecid decreased the risk of CVD, including HF exacerbation (42). But one study showed that compare to allopurinol in the same uric acid decreasing, probenecid could not improve endothelial-dependent vasodilatation (39). Probenecid promotes serum uric acid excretion through the kidneys and causes the inhibition of pannexin 1 channels and IL-1b. These inhibitions have been hypothesized to have potential beneficial effects on CVD. At present, however, using uricosuric drugs for a cardioprotective effect remains controversial (26,40). Our findings showed no benefits in HF.
Combination therapy
In our study, treating gout with combination therapy was found to worsen HF incidence. Our explanations for this outcome are as follows. First, poor compliance with or adherence to gout treatment (29,30) might have caused the higher levels or fluctuations of uric acid and gout flare, inducing persistent low-grade inflammation and HF. Second, combination therapy is associated with cases with a more severe or longer duration of gout treatment, which is concordant with previous findings regarding the association between gout and HF (18,27). Third, we need more basic research on the drug-drug interaction of these types of antigout medications in gout treatment and HF.
Limitations
First, we could not obtain additional laboratory data, such as uric acid levels, images of joints, heart echoes, or functional evaluations, due to national principal restrictions. Second, we could not identify all other potential confounding factors in our database, such as smoking, alcohol consumption, or the nutritional status and environmental factors of the patients.
Conclusions
In Taiwan, the prevalence of hyperuricemia has decreased, but gout has increased gradually (5). To our knowledge, gout and hyperuricemia are not synonymous and have distinct pathophysiologic pathways. However, the inflammatory activity of gout might induce more CVD, such as HF. Our study revealed that gout treatment in Taiwan cannot improve HF and actually increase the risk for HF after combination therapy for gout. However, according to the results of our study, from the public health point of view, it is easy for patients with gout flare to obtain medical help and treatment for painful joints by primary physicians. This suggests that we should pay more attention to the gout treatment burden to decrease HF incidence.
Acknowledgments
Funding: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW108-TDU-B-212-133004); China Medical University Hospital (DMR-107-192, CMU107-ASIA-19); Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 108-2321-B-039-003-); Tseng-Lien Lin Foundation, Taichung, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.124). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was exempted from a full ethical review by China Medical University and Hospital Research Ethics Committee (IRB permit number: CMUH104-REC2-115-R4).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang HY, Pan WH, Yeh WT, et al. Hyperuricemia and gout in Taiwan: results from the Nutritional and Health Survey in Taiwan (1993-96). J Rheumatol 2001;28:1640-6. [PubMed]
- Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999;131:7-13. [Crossref] [PubMed]
- Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA 2000;283:2404-10. [Crossref] [PubMed]
- Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62:170-80. [PubMed]
- Chuang SY, Lee SC, Hsieh YT, et al. Trends in hyperuricemia and gout prevalence: Nutrition and Health Survey in Taiwan from 1993-1996 to 2005-2008. Asia Pac J Clin Nutr 2011;20:301-8. [PubMed]
- Choi HK, Atkinson K, Karlson EW, et al. Obesity, weight change hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005;165:742-8. [Crossref] [PubMed]
- Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med 2012;125:679-87.e1. [Crossref] [PubMed]
- Chen JH. A Prospective Study of Hyperuricemia on Gouty Arthritis and Mortality of Cardiovascular Disease. PhD dissection. 2009.
- Krishnan E, Svendsen K, Neaton JD, et al. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med 2008;168:1104-10. [Crossref] [PubMed]
- Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894-900. [Crossref] [PubMed]
- Kuo CF, See LC, Luo SF, et al. Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology (Oxford) 2010;49:141-6. [Crossref] [PubMed]
- Spieker LE, Ruschitzka FT, Lüscher TF, et al. The management of hyperuricemia and gout in patients with heart failure. Eur J Heart Fail 2002;4:403-10. [Crossref] [PubMed]
- Hueskes BA, Willems FF, Leen AC, et al. A case-control study of determinants for the occurrence of gouty arthritis in heart failure patients. Eur J Heart Fail 2012;14:916-21. [Crossref] [PubMed]
- Dzudie A, Kengne AP, Mbahe S, et al. Chronic heart failure, selected risk factors and co-morbidities among adults treated for hypertension in a cardiac referral hospital in Cameroon. Eur J Heart Fail 2008;10:367-72. [Crossref] [PubMed]
- Krishnan E. Hyperuricemia and incident heart failure. Circ Heart Fail 2009;2:556-62. [Crossref] [PubMed]
- Holme I, Aastveit AH, Hammar N, et al. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med 2009;266:558-70. [Crossref] [PubMed]
- Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ Open 2012;2:e000282. [Crossref] [PubMed]
- Stack AG, Hanley A, Casserly L, et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM 2013;106:647-58. [Crossref] [PubMed]
- Kuo CF, Grainge MJ, Mallen C, et al. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis 2015;74:661-7. [Crossref] [PubMed]
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 2011;63:3136-41. [Crossref] [PubMed]
- Chou CT, Lai JS. The epidemiology of hyperuricaemia and gout in Taiwan aborigines. Br J Rheumatol 1998;37:258-62. [Crossref] [PubMed]
- Kuo CF, Grainge MJ, See LC, et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther 2015;17:13. [Crossref] [PubMed]
- Antón FM, García Puig J, Ramos T, et al. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism 1986;35:343-8. [Crossref] [PubMed]
- Disveld IJM, Fransen J, Rongen GA, et al. Crystal-proven Gout and Characteristic Gout Severity Factors Are Associated with Cardiovascular Disease. J Rheumatol 2018;45:858-63. [Crossref] [PubMed]
- Bhole V, Krishnan E. Gout and the heart. Rheum Dis Clin North Am 2014;40:125-43. [Crossref] [PubMed]
- Thanassoulis G, Brophy JM, Richard H, et al. Gout, allopurinol use, and heart failure outcomes. Arch Intern Med 2010;170:1358. [Crossref] [PubMed]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Vazirpanah N, Kienhorst LBE, Van Lochem E. Patients with gout have short telomeres compared with healthy participants: association of telomere length with flare frequency and cardiovascular disease in gout. Ann Rheum Dis 2017;76:1313-9. [Crossref] [PubMed]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999;340:115-26. [Crossref] [PubMed]
- Doherty M, Jansen TL, Nuki G, et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis 2012;71:1765-70. [Crossref] [PubMed]
- Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med 1984;310:563-72. [Crossref] [PubMed]
- Ravelli RB, Gigant B, Curmi PA, et al. Insight into tubulin regulation from a complex with colchicine and a stathminlike domain. Nature 2004;428:198-202. [Crossref] [PubMed]
- Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404-10. [Crossref] [PubMed]
- Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019;381:2497-505. [Crossref] [PubMed]
- Kinugawa S, Tsutsui H, Hayashidani S, et al. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 2000;87:392-8. [Crossref] [PubMed]
- Landmesser U, Spiekermann S, Dikalov S, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine oxidase and extracellular superoxide dismutase. Circulation 2002;106:3073-8. [Crossref] [PubMed]
- White WB, Saag KG, Becker MA, et al. Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. N Engl J Med 2018;378:1200-10. [Crossref] [PubMed]
- Struthers AD, Donnan P, Lindsay P, et al. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart 2002;87:229-34. [Crossref] [PubMed]
- George J, Carr E, Davies J, et al. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 2006;114:2508-16. [Crossref] [PubMed]
- Neogi T. Clinical practice. Gout. N Engl J Med 2011;364:443-52. [Crossref] [PubMed]
- Kim SC, Neogi T, Kang EH, et al. Cardiovascular Risks of Probenecid Versus Allopurinol in Older Patients With Gout. J Am Coll Cardiol 2018;71:994-1004. [Crossref] [PubMed]
- Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 2012;126:2739-48. [Crossref] [PubMed]


