
Aspirin use and endometrial cancer risk: a meta-analysis and systematic review
Introduction
Endometrial cancer is the most common cancer in the female reproductive system in the USA. Also, it is the fourth most common malignancy for women after breast, lung, and colorectal cancer (1). Obesity, tamoxifen, polycystic ovarian syndrome, and diabetes are all well-defined risk factors for endometrial cancer. These risk factors are closely related to unopposed estrogen and chronic inflammation. Estrogen, unopposed by progesterone, can stimulate cell proliferation and increase the risk of malignant transformation. Chronic inflammation can promote cell division, and induce DNA damage and mutations, which provokes the carcinogenesis (2,3). Furthermore, excess estrogens can induce a pro-inflammatory milieu in the endometrium and, in turn, this pro-inflammatory milieu can also directly increase estrogen production. Hence, inflammation may work in conjunction with or in addition to excess estrogen exposure in the development of endometrial cancer (2).
Aspirin, also known as acetylsalicylic acid, is one of the most widely used nonsteroidal anti-inflammatory drugs (NSAIDs) in the world. The mechanisms of the anti-inflammation and anti-platelet aggregation ability of aspirin is predominantly considered to be its irreversible inactivation of cyclooxygenase (COX) (4). Platelets play an essential role in inflammation through recruiting leucocytes by releasing many factors, including proteins (such as angiogenic and growth factors) and lipids (such as thromboxane), and adenosine diphosphate stored in granules. This platelet activation and the pro-inflammatory microenvironment are believed to contribute to tumorigenesis and cancer metastasis (5).
Therefore, in the current literature, aspirin has been gaining notable attention due to its tumor-preventive effects (6). There is increasing evidence suggesting there to be an association between aspirin use and reduced risk of several common cancers, particularly of gastrointestinal tract cancers (7). In vitro studies have shown that aspirin suppresses the proliferation and induces the apoptosis of endometrial cancer cell lines in a dose-dependent manner (8,9). Furthermore, the association between aspirin use and endometrial cancer risk has been examined in several observational epidemiological studies and meta-analyses (10-12). However, the inconsistent findings form these studies have rendered knowledge concerning the chemopreventive effect of aspirin for endometrial cancer unclear in the public eye. Given that no meta-analysis thus far has included all the relevant studies and considering the limitations of the analytical methods in some of the literature, we conducted our own review to clarify this ambiguity. We identified 7 case-control studies and 11 cohort studies after searching electronic databases for all the latest evidence, and then performed a meta-analysis and subsequent subgroup analysis based on the frequency and duration of aspirin use and obesity using a reliable method discussed below.
Methods
Literature search and study selection
We conducted a systematic literature search using the following electronic databases: PubMed, EMBASE, and Web of Science (last search: December 10, 2019). Controlled vocabularies and keywords were used in the search strategy (Supplementary), and we reviewed the reference lists of closely related articles to obtain more eligible articles. For the studies to be included in the analysis: (I) they needed to be a randomized controlled trial (RCT), case-control or cohort study; (II) they need to be evaluate the association between aspirin use and risk of endometrial cancer; and (III) the association needed to be presented as odds ratio (OR) or relative risk (RR) (including hazard ratio) with corresponding confidence interval (CI). Studies controlling for aspirin use in statistical models without numerically reporting effect measures or studies based on individuals with a predisposition for endometrial cancer were eliminated. The details of the study selection are presented in a flow chart (Figure 1). The ethics committee of the Obstetrics & Gynecology Hospital of Fudan University approved our study.
Data extraction and statistical analysis
In the process of study evaluation, we referred to the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (13) and the Newcastle-Ottawa Quality Assessment Scale for observational studies (14). Two authors independently selected studies, extracted data, and evaluated the quality of the studies. Any discrepancies were resolved by discussion between all authors. For each eligible study, we extracted key characteristics (e.g., study time, setting, measurement, study design, and adjusted confounders) (Tables 1 and 2). In the overall analysis, any use of aspirin was compared to non-use after pooled the risk estimates and corresponding 95% CIs. When more than one type of estimates was presented in the article, only the estimate adjusted for the most covariates was adopted. When the data of more than one exposure level of a category were available, we did not choose the result of the highest rank or the level with statistical significance like others, but instead calculated the corresponding estimates of the whole category using the method proposed by Hamling et al. (33). This method was used to combine estimates using the same reference category or the same set of controls, taking correlations between levels into account. Subsequently, sensitivity and subgroup analysis, restricted to the subgroup contained in ≥5 studies, were performed. In the sensitivity and subgroup analysis based on the longest duration of aspirin use, the maximum frequency of aspirin use, and obesity (BMI >30), we pooled the ORs and RRs together because of the low prevalence of endometrial cancer and the limited number of included studies (34).

Full table

Full table
Using the random effects model, we pooled the ORs (case-control studies) and RRs (cohort studies), respectively in the overall analysis, and merged the ORs and RRs in the sensitivity and subgroup analysis. Cochrane Q test was used to assess the statistical heterogeneity in the meta-analysis. We rated the statistical heterogeneity as substantial if the I2 was >50.0% and P was >0.05. To investigate the publication bias, we first visually inspected funnel plots for obvious asymmetry and then performed a quantitative assessment using the Egger test (35). The existence of publication bias was indicated by a P values of less than 5%. All statistical analyses were conducted in Stata 15.0 (StataCorp, LLP, College Station, TX, USA).
Results
After searching the electronic databases, evaluating the abstracts, and reviewing the full-text of some of the key articles, we finally identified 7 case-control studies (15-21) and 11 cohort studies (22-32) but no eligible RCTs. In 5 of these studies (22-24,29,32), the data of the association between aspirin use and endometrial cancer risk were not provided in the original articles but were published and pooled in one study of the Epidemiology of Endometrial Cancer Consortium (E2C2) (12), in which all cohort studies are analyzed as nested case-control studies. Because nested case-control studies possessed more powerful statistical efficiency than that of case-control studies and, to some extent, indicated a causal relationship as cohort studies, we pooled the data of these 5 nested case-control-analyzed studies and other cohort studies together. Also, we adopted the data of 1 case-control study from E2C2, because it had more adjusted variables than that of the original study (16).
Study characteristics
The included studies involved a total of 14,766 endometrial cancer cases and were conducted within an extended period [1979–2012]. The majority of studies conducted were from the USA, with one from Italy (18), one from Australia (20), one from Denmark (21), and one from in Sweden (32). In these studies, almost all the exposure assessments, including medication use, were obtained through interviews or questionnaires; the exceptions were the Danish study (21) and the Swedish study (32), both of which used the national registration system to acquire information about aspirin use. There was a clear heterogeneity in definitions of aspirin use, and the frequency and the duration of aspirin use. The concise characteristics of included studies are summarized in Tables 1 and 2. Although many studies tried to assess the role of both aspirin use and non-aspirin NSAID use in the prevention of endometrial cancer, we focused our interest in the former due to the diverse types of the latter.
Overall analysis
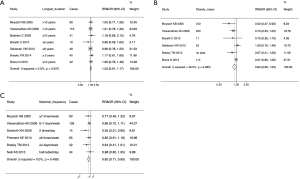
Results of the general analysis for any aspirin use versus non-use are presented in Figure 2. The data shows that there was no substantial heterogeneity among the studies (I2<50%, P>5%) but an inverse association between aspirin use and endometrial cancer risk was demonstrated in both case-control studies (pooled ORs =0.88, 95% CI: 0.78–0.98) and cohort studies (pooled RRs =0.86, 95% CI: 0.86–0.99). Funnel plots and the Egger test did not show significant publication bias (P=0.092 for case-control studies and P=0.069 for cohort studies, Figure S1). When we pooled the data of the case-control studies and cohort studies together, the negative relationship between aspirin use and endometrial cancer risk remained (pooled RRs & ORs =0.92, 95% CI: 0.88–0.97). However, publication bias was noticed using the Egger test (P<0.05, data not shown).


Subgroup and sensitivity analysis
In the subgroup and sensitivity analysis, we pooled the data of case-control studies and cohort studies together and evaluated the effects of the highest frequency and the longest duration of aspirin use and obesity on the association of aspirin use and endometrial cancer risk. The results indicate that the longest duration of aspirin use and obese women who use aspirin did not exhibit a reduced endometrial cancer risk (pooled RRs & ORs: 1.03, 95% CI: 0.91–1.17 and pooled RRs & ORs: 0.84, 95% CI: 0.69–1.03, respectively; Figure 3A,B). However, the longest frequency of aspirin use was linked to reduce the risk of endometrial cancer (pooled RRs & ORs: 0.82, 95% CI: 0.71–0.95; Figure 3C) without significant publication bias (P=0.16, Figure S2).

Discussion
Based on the overall meta-analysis of 7 case-control studies and 11 cohort studies, we observed that aspirin use was statistically associated with 12% reduction of endometrial cancer risk in the case-control studies (pooled ORs: 0.88, 95% CI: 0.78–0.98). Correspondingly, there was an 8% reduced risk in the cohort studies (pool RRs: 0.92, 95% CI: 0.86–0.99), which was similar to the results of Zhang et al.’s study (11), which included 6 case-control studies and 6 cohort studies (pooled RRs & ORs: 0.93, 95% CI: 0.88–0.99). These results were different to the statistically non-significant result of Verdoodt et al. which contained 6 case-control studies and 7 cohort studies (pooled ORs: 0.89, 95% CI: 0.79–1.01 and pooled RRs: 0.92, 95% CI: 0.84–1.00) (10). Compared with the borderline inverse association in Webb et al.’s studies in 2019, which analyzed 5 case-control studies and 7 cohort studies (pooled ORs: 0.85, 95% CI: 0.79–1.00 and pooled RRs: 0.93, 95% CI: 0.88–1.05; overall: 0.93, 95% CI: 0.86–1.00) (12), the present meta-analysis, include the largest number of both case-control studies (7) and cohort studies (11), and demonstrated a negative association between aspirin use and endometrial cancer risk.
Also, one study (36) pooled in the meta-analysis of both Verdoodt et al. and Zhang et al. was excluded from our analysis because the authors merely evaluated the association of aspirin use and all forms of cancers in uterine body. In Danforth et al.’s and Brasky et al.’s cohort studies, the estimations were divided into inconsistent use and consistent use groups while only the RRs of current aspirin use were pooled into the meta-analysis of both the studies of Verdoodt et al. (10) and Zhang et al. (11). Nevertheless, we conducted the analysis based on the comparison of general aspirin use with non-use through the method provided by Hamling et al. (33), which might have potentially improved the reliability of our meta-analysis. Furthermore, in the meta-analysis of Verdoodt et al. and Zhang et al., some subgroup and sensitivity analyses were composed of too few studies (≤3), and some analyses were based on the risk estimation of a single level of a category rather than the whole. To decrease the possible biases, we only pooled the subgroups incorporated in the analysis of ≥5 studies and conducted the sensitivity and subgroup analysis according to general OR or RR of one category as far as possible. In the present meta-analysis, we found that there was no significant influence of the longest duration of aspirin on endometrial cancer risk and that obese women who used aspirin did not exhibit significant reduced endometrial cancer risk (pooled RRs & ORs: 1.03, 95% CI: 0.91–1.17 and pooled RRs & ORs: 0.84, 95% CI: 0.69–1.03, respectively; Figure 3A,B). However, we could observe a statistically significant reduced risk based on the maximum frequency of aspirin use (pooled RRs & ORs: 0.82, 95% CI: 0.71–0.95; Figure 3C). Furthermore, similar to any other meta-analysis, the definitions of aspirin use were heterogeneous, while in our meta-analysis the statistical significance was marginal. Therefore, the small reduction in risk should be interpreted cautiously, especially when applying conclusions to a clinical situation.
It is acceptable that the more frequently the medicine is taken, the longer the effective concentration of the drug will remain, and this is essential to exerting a pharmacological effect. In this sense, it may not be surprising to notice that frequency rather than the duration of aspirin use was more closely related to endometrial cancer prevention. Also, it is reasonable to presume that aspirin use in obese women, who were in a chronic low-grade inflammatory state, could decrease the endometrial cancer risk as demonstrated in the meta-analysis of Zhang et al. (11) (pooled RRs & ORs: 0.83, 95% CI: 0.69–0.99) and Webb et al. (pooled RRs & ORs: 0.86, 95% CI: 0.76–0.97) (12). However, we failed to confirm this assumption (pooled RRs & ORs: 0.84, 95% CI: 0.69–1.03). This may be partly due to the possible heterogeneity and the limited number of our included studies (I2=0.482, Figure 3B).
There is more clinical data available for low-dose aspirin use than for regular-dose, because of the wide use of low-dose aspirin in the prevention and treatment of cardiovascular disease. However, it is known that low-dose and regular-dose aspirin have different physiologic effects (37). Among the studies in our meta-analysis, only 2 pieces of research clearly defined the dose of aspirin use (21,30); therefore, we pooled the data of aspirin use together regardless of the dosage and could not do a stratification analysis based on dosage alone. This might have partly contributed to the marginal effect of aspirin use, as shown in our results (Figure 2). Also, it is understandable and has been confirmed that the emergence of protective effects of aspirin on cancer may require a prolonged duration of use range from 5 years to 20 years (8,38,39). Our results, however, indicated that the longest duration of aspirin use (at least ≥5 years) did not decrease the endometrial cancer risk (1.03, 95% CI: 0.91–1.17). The reason for this lack of effect was unclear. One hypothesis is that the small number of users in each longest duration subgroup might have masked the potential beneficial effects. As a result, further investigation to evaluate the duration, frequency, and dose of aspirin use on the occurrence of endometrial cancer is warranted.
Potential mechanisms through which aspirin exerts anti-cancer action are subject to active investigations. In the development of cancer, inflammation has been proven to be critically involved. COX is the limiting enzyme in the production of pro-inflammatory cytokines. Therefore, it is reasonable to deduce that aspirin may exert an anticancer effect via inhibition of COX. One of the most accepted anti-cancer mechanisms of aspirin is through COX-2/PGE2 inhibition. It is shown that PGE2 can promote endometrial tumorigenesis by enhancing small ubiquitin-related modifier-1 activity and inactivating the tuberin (40,41). Also, PGE2 can stimulate aromatase expression and thereby increase estrogen production (42).
Nevertheless, excess estrogen is a well-known risk factor for endometrial cancer in obesity or post-menopausal women. Therefore, aspirin may reduce estrogen biosynthesis via the inhibition of the COX-2/PGE2 pathway and provide a potential protective mechanism. On the other hand, it has been suggested that inhibition of platelet COX-1 activity contributes to the chemopreventive effects of aspirin in colorectal cancer (43,44), and low-dose aspirin can reduce 55% systemic basal PGE2 biosynthesis in healthy females (45). Therefore, the platelet activation induced by chronic inflammation may be blocked via the inhibition of COX-1 by aspirin use, which is likely to slow down the oncogenesis of endometrial cancer. However, these notions require confirmation through further study.
Recently, emerging evidence has demonstrated that aspirin use not only decreases the risk of cancer but also improves the survival of cancer patients (46). However, for aspirin use and the survival of endometrial cancer, the evidence is limited and the conclusions are inconsistent. In a multicenter retrospective study, the result suggested that low-dose aspirin use improved the survival outcomes of women with endometrial cancer (47). In contrast, a population-based cohort study and a nationwide study found no significant associations between aspirin use and endometrial cancer survival (48,49). In another large cohort study, aspirin use for >10 years showed an approximately two-fold increased risk of type II endometrial cancer-specific mortality (50). The reason for these contradictions is elusive. Different populations and tumor characteristics in these studies may account for this confusion. More investigations should be done before convincing conclusions can be made.
Our analysis has some methodological strengths. First, we incorporated the current largest number of studies after searching the databases and reading the references of core articles. We included 5 studies (22-24,29,32) that had not provided the data about aspirin use and endometrial cancer risk in original articles but these data were included in a recent high-quality meta-analysis (12). Meanwhile, we excluded 1 inappropriate study included in the previous review (11). Second, when a group contained several levels of a single category, we calculated the entirety of the estimation using a novel and reliable method introduced by Hamling et al. (33), instead of adopting the significant or partial result from one level. It might have helped to reduce favorable bias. Therefore, to some extent, the present studies may be more comprehensive and reliable.
The limitations present in our study are mainly due to the nature of observational studies. First, in general, observational studies may be more prone to having selection and recall bias, and some methodological heterogeneities inevitably exist. For instance, the definition of aspirin use, frequency, and duration of aspirin use are different across studies. Second, some critical parameters, such as body mass index and patterns of aspirin use change during the follow-up period and few studies have attempted to tackle these defects in analyses. Third, although the ORs of case-control studies and RRs of cohort studies could be pooled together statistically due to the rare incidence of endometrial cancer in the general population, this might have distorted the real relationship. However, most of the participants in the included studies were postmenopausal women (Tables 1 and 2), which is likely to have masked the beneficial effect in some studies and undermined our ability to discover the salutary effect. Last but not least, we included no RCTs, and these are highly weighted types of research in evidence-based medicine.
In conclusion, we observed a reduced risk of endometrial association with aspirin use. The association was statistically significant in the maximal frequency of aspirin use but not in the longest duration of aspirin use or obese women. However, as always, extrapolating this result to clinical application should done with caution, and more relevant RCTs should be conducted in the future.
Supplementary
Search strategy
PubMed
("Aspirin" [Mesh] OR "Aspirin" [tw]) AND ("Uterus"[MeSH] OR Endometri* OR Uteri* OR "uterus" [tw]) AND ("Neoplasms"[Mesh] OR Neoplas* OR "Tumor" OR "Tumour" OR "Cancer" OR Carcinogen* OR Tumorigen* OR Oncogen* OR sarcoma* OR malignan* OR adenocarcinoma* OR "tumors" [tw] OR "tumours" [tw] OR "cancers" [tw])
Web of Science
TS = ((neoplas* OR "tumor" OR "tumour" OR "cancer" OR carcinoma* OR sarcoma* OR adenocarcinoma* OR adenosarcoma*) AND (uteri* OR "uterus" OR endometri*) AND ("Aspirin"))
Embase
('neoplasm'/exp OR neoplas* OR 'tumor' OR 'tumour' OR 'cancer' OR carcinoma* OR sarcoma* OR adenocarcinoma* OR adenosarcoma*) AND ('uterus'/exp OR uteri* OR 'uterus' OR endometri*) AND ('aspirin'/exp OR 'aspirin')
Acknowledgments
Funding: This work was supported by the Shanghai Committee of Science and Technology, China (SCST 15411964700), and the National Natural Science Foundation of China (NSFC 81572555).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.125). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31-54. [Crossref] [PubMed]
- Modugno F, Ness RB, Chen C, et al. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev 2005;14:2840-7. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- McQuillan A, Eikelboom JW. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2002;346:1589-90; author reply 1590. [Crossref] [PubMed]
- Dovizio M, Alberti S, Guillem-Llobat P, et al. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin Pharmacol Toxicol 2014;114:118-27. [Crossref] [PubMed]
- Li D, Wang P, Yu Y, et al. Tumor-preventing activity of aspirin in multiple cancers based on bio-informatic analyses. Peer J 2018;6:e5667. [Crossref] [PubMed]
- Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 ran-domised controlled trials. Lancet 2012;379:1602-12. [Crossref] [PubMed]
- Arango HA, Icely S, Roberts WS, et al. Aspirin effects on endometrial cancer cell growth. Obstet Gynecol 2001;97:423-7. [PubMed]
- Gao J, Niwa K, Sun W, et al. Non-steroidal anti-inflammatory drugs inhibit cellular proliferation and upregulate cyclooxygenase-2 protein expression in endometrial cancer cells. Cancer Sci 2004;95:901-7. [Crossref] [PubMed]
- Verdoodt F, Friis S, Dehlendorff C, et al. Non-steroidal anti-inflammatory drug use and risk of endometrial cancer: A systematic review and meta-analysis of observational studies. Gynecol Oncol 2016;140:352-8. [Crossref] [PubMed]
- Zhang D, Bai B, Xi Y, et al. Can Aspirin Reduce the Risk of Endometrial Cancer?: A Systematic Review and Meta-analysis of Observational Studies. Int J Gynecol Cancer 2016;26:1111-20. [Crossref] [PubMed]
- Webb PM, Na R, Weiderpass E, et al. Use of aspirin, other nonsteroidal anti-inflammatory drugs and acetaminophen and risk of endometrial cancer: the Epidemiology of Endometrial Cancer Consortium. Ann Oncol 2019;30:310-6. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195. [Crossref] [PubMed]
- Moysich KB, Baker JA, Rodabaugh KJ, et al. Regular analgesic use and risk of endometrial can-cer. Cancer Epidemiol Biomarkers Prev 2005;14:2923-8. [Crossref] [PubMed]
- Fortuny J, Sima C, Bayuga S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomarkers Prev 2009;18:1448-56. [Crossref] [PubMed]
- Bodelon C, Doherty JA, Chen C, et al. Use of nonsteroidal antiinflammatory drugs and risk of endometrial cancer. Am J Epidemiol 2009;170:1512-7. [Crossref] [PubMed]
- Bosetti C, Bravi F, Talamini R, et al. Aspirin and risk of endometrial cancer: a case-control study from Italy. Eur J Cancer Prev 2010;19:401-3. [Crossref] [PubMed]
- Lu L, Risch H, Irwin ML, et al. Long-term overweight and weight gain in early adulthood in as-sociation with risk of endometrial cancer. Int J Cancer 2011;129:1237-43. [Crossref] [PubMed]
- Neill AS, Nagle CM, Protani MM, et al. Aspirin, nonsteroidal anti-inflammatory drugs, parace-tamol and risk of endometrial cancer: a case-control study, systematic review and me-ta-analysis. Int J Cancer 2013;132:1146-55. [Crossref] [PubMed]
- Brons N, Baandrup L, Dehlendorff C, et al. Use of nonsteroidal anti-inflammatory drugs and risk of endometrial cancer: a nationwide case-control study. Cancer Causes Control 2015;26:973-81. [Crossref] [PubMed]
- Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc (1972) 1995;50:56-8. [PubMed]
- Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273s-309s. [Crossref] [PubMed]
- Lacey JV Jr, Brinton LA, Lubin JH, et al. Endometrial carcinoma risks among menopausal estro-gen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev 2005;14:1724-31. [Crossref] [PubMed]
- Viswanathan AN, Feskanich D, Schernhammer ES, et al. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res 2008;68:2507-13. [Crossref] [PubMed]
- Danforth KN, Gierach GL, Brinton LA, et al. Nonsteroidal anti-inflammatory drug use and en-dometrial cancer risk in the NIH-AARP Diet and Health Study. Cancer Prev Res (Phila) 2009;2:466-72. [Crossref] [PubMed]
- Prizment AE, Folsom AR, Anderson KE. Nonsteroidal anti-inflammatory drugs and risk for ovarian and endometrial cancers in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev 2010;19:435-42. [Crossref] [PubMed]
- Setiawan VW, Matsuno RK, Lurie G, et al. Use of nonsteroidal anti-inflammatory drugs and risk of ovarian and endometrial cancer: the Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev 2012;21:1441-9. [Crossref] [PubMed]
- Yang HP, Wentzensen N, Trabert B, et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. Am J Epidemiol 2013;177:142-51. [Crossref] [PubMed]
- Brasky TM, Moysich KB, Cohn DE, et al. Non-steroidal anti-inflammatory drugs and endome-trial cancer risk in the VITamins And Lifestyle (VITAL) cohort. Gynecol Oncol 2013;128:113-9. [Crossref] [PubMed]
- Brasky TM, Liu J, White E, et al. Non-steroidal anti-inflammatory drugs and cancer risk in women: results from the Women's Health Initiative. Int J Cancer 2014;135:1869-83. [Crossref] [PubMed]
- Roswall N, Sandin S, Adami HO, et al. Cohort Profile: The Swedish Women's Lifestyle and Health cohort. Int J Epidemiol 2017;46:e8. [Crossref] [PubMed]
- Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by expo-sure level or disease category. Stat Med 2008;27:954-70. [Crossref] [PubMed]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1-30. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 1994;5:138-46. [Crossref] [PubMed]
- Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 2009;10:501-7. [Crossref] [PubMed]
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer inci-dence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741-50. [Crossref] [PubMed]
- Bibbins-Domingo K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Col-orectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836-45. [Crossref] [PubMed]
- Sales KJ, Battersby S, Williams AR, et al. Prostaglandin E2 mediates phosphorylation and down-regulation of the tuberous sclerosis-2 tumor suppressor (tuberin) in human endometrial adenocarcinoma cells via the Akt signaling pathway. J Clin Endocrinol Metab 2004;89:6112-8. [Crossref] [PubMed]
- Ke J, Yang Y, Che Q, et al. Prostaglandin E2 (PGE2) promotes proliferation and invasion by en-hancing SUMO-1 activity via EP4 receptor in endometrial cancer. Tumour Biol 2016;37:12203-11. [Crossref] [PubMed]
- Zhao Y, Agarwal VR, Mendelson CR, et al. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aroma-tase) gene. Endocrinology 1996;137:5739-42. [Crossref] [PubMed]
- Dovizio M, Bruno A, Tacconelli S, et al. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res 2013;191:39-65. [Crossref] [PubMed]
- Sciulli MG, Filabozzi P, Tacconelli S, et al. Platelet activation in patients with colorectal cancer. Prostaglandins Leukot Essent Fatty Acids 2005;72:79-83. [Crossref] [PubMed]
- Boutaud O, Sosa IR, Amin T, et al. Inhibition of the Biosynthesis of Prostaglandin E2 By Low-Dose Aspirin: Implications for Adenocarcinoma Metastasis. Cancer Prev Res (Phila) 2016;9:855-65. [Crossref] [PubMed]
- Rothwell PM, Fowkes FGR, Belch JFF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet (London, England) 2011;377:31-41. [Crossref] [PubMed]
- Matsuo K, Cahoon SS, Yoshihara K, et al. Association of Low-Dose Aspirin and Survival of Women With Endometrial Cancer. Obstet Gynecol 2016;128:127-37. [Crossref] [PubMed]
- Sanni OB, Mc Menamin ÚC, Cardwell CR, et al. Commonly used medications and endometrial cancer survival: a population-based cohort study. Br J Cancer 2017;117:432-8. [Crossref] [PubMed]
- Sperling CD, Verdoodt F, Aalborg GL, et al. Low-dose aspirin use and endometrial cancer mortality-a Danish nationwide cohort study. Inter J Epidemiol 2019:dyz253.
- Brasky TM, Felix AS, Cohn DE, et al. Nonsteroidal Anti-inflammatory Drugs and Endometrial Carcinoma Mortality and Recurrence. J Natl Cancer Inst 2017;109:1-10. [Crossref] [PubMed]



