
Development of new poly(ADP-ribose) polymerase (PARP) inhibitors in ovarian cancer: Quo Vadis?
Introduction
Approximately 22,440 newly diagnosed cases of ovarian cancer and 14,080 deaths occurred in the United States in 2017 (1). Two thirds of patients present at advanced stages, whilst the estimated 5-year survival rate is 20–40%. The vast majority of ovarian cancers are epithelial in origin (90%), whereas 10% are non-epithelial; germ cell and sex cord stromal cell (5% each). They differ in epidemiology, etiology, and treatment. Epithelial ovarian cancer (EOC) is the most frequent cause of death from gynecologic cancer among women due to lack of an effective screening test. Histologically, it is predominantly divided into five main subtypes; high- and low-grade serous (75–80%), endometrioid and clear cell (10% each), and mucinous (3%) (2). Patients with EOC response usually well to the initial standard treatment, which includes cytoreductive surgery with either preoperative or adjuvant platinum-based chemotherapy; nevertheless, the estimated median progression-free survival (PFS) ranges from 12 to 18 months (3). Therefore, development and validation of functional biomarkers and novel therapeutic agents are of major importance for the improvement of patients’ outcome.
Breast cancer genes 1 and 2 (BRCA1/2) mutations are the most significant molecular aberrations in ovarian cancer with established prognostic and predictive value following chemotherapy. Based on that, increased research focused on germline variant testing, risk stratification, early detection, and cancer prevention for BRCA1/2 mutation carriers has been conducted (4). Cells with mutations in BRCA1/2 genes have an impaired double-strand DNA breaks (DSBs). In any case of impairment of homologous recombination (HR), synthetic lethality induced by poly (ADP-ribose) polymerase (PARP) inhibition occurs and may target tumor tissue selectively (5,6). Furthermore, several somatic mutations beyond BRCA1/2 genes have been recognized, including RAD51, and ataxia telangiectasia-mutated (ATM), which are also involved in HR repair (7). Tumors with these abnormalities are often sensitive to similar therapies (8).
Over the last decade, clinical trials led to the approval of several PARP inhibitors in ovarian cancer. Olaparib, rucaparib, and niraparib have all obtained US Food and Drug Administration (FDA) and/or European Medicines Agency (EMA) approval in EOC in different settings. Veliparib and talazoparib are in earlier clinical development. Veliparib was evaluated mainly combined with chemotherapy or targeted agents (9), whilst at least in vitro talazoparib demonstrates more potent antitumor activity, based on its enhanced PARP-DNA trapping ability (10).
The purpose of this article is to review the mechanisms of HR, and provide current evidence and future challenges in the development of the investigational PARP inhibitors veliparib and talazoparib.
Mechanisms of DNA repair
DNA damage often arises within the context of normal cellular processes. It can be spontaneous or caused by cell metabolism or by environmental agents (11). Base excision repair (BER) is the major DNA repair pathway responsible for the removal of DNA base damage and formation of single-strand DNA breaks (SSBs) and DSBs. The primary activity of PARP1/2 proteins, is post-translational poly-ADP ribosylation (PARylation) of substrate proteins involved in biological processes such as transcription and DNA damage repair. The idea of PARylation asserts that during DNA damage PARP1 is activated, on both SSB and DSB. In addition, several post-translational modifications also alter activity of PARP1, which is implicated in multiple signaling pathways (12). Once PARP is activated, downstream events of PARP signaling take place, involving either covalent PARylation of substrates, non-covalent binding of PAR polymer to proteins bearing a PAR-binding motif, liberation of free PAR to the cell or lowering cellular NAD+/ATP levels. This could lead to loss of genomic instability, cell death, and even carcinogenesis if not correctly repaired (13). HR and non-homologous end-joining (NHEJ) likely playing the largest role in DSB repair. How the cell determines whether HR or NHEJ will be used to repair a break depends on the phase of the cell cycle. HR predominates as a mechanism of repair during mid S and G2 phases (14). If an undamaged template DNA is unavailable, then the faster but error-prone NHEJ repair pathway is the primary method of DNA DSB repair in the cell (15). Additional DNA damage repair operational mechanisms include nucleotide excision repair (NER), mismatch repair (MMR), and translesional synthesis (16). In the presence of functional defects of both HR and classical NHEJ, inhibition of PARP1 inhibits alternative NHEJ, resulting in cell apoptosis (17).
Seventeen members of the PARP proteins have been described so far. PARP1 is responsible for approximately 90% of the PARylation activity, whereas PARP2 and to a lesser extent PARP3 function in fewer, but overlapping DNA repair processes (18). Binding of PARP to damaged sites, its catalytic activity, and its eventual release from DNA are key elements for potential response of a cancer cell to DNA breaks introduced by certain chemotherapeutic agents, and radiation (19). When activated PARP, it recruits other DNA repair proteins (20).
PARP inhibition and synthetic lethality
Two preclinical studies published in 2005 promoted knowledge and clinical development of PARP inhibitors (5,6). In view of assessing the effects of PARP1 depletion, a plasmid expressing a short interfering RNA targeting mouse PARP1 was transfected into embryonic stem cells lacking wild-type BRCA1/2. These cells bared specific genomic mutations of BRCA1/2, lacked wild-type single allele and were directly compared to their isogenic wild-type counterparts. Investigators concluded that these cell lines with a BRCA1/2 mutation were more sensitive to PARP inhibition than heterozygous mutants. This was based on the synthetic lethality, characterized by a bimodal dependency through which the loss of function of one gene in a cell does not have impact on viability, whilst the combined loss of both components results in cell death (21). The synthetic lethality between PARP inhibition and HR deficiency is overall produced due to SSBs repair failure. If the inhibitor stays bound within the PARP active site and the PARP protein is trapped on the DNA long enough to be encountered by the replication machinery, this can lead in a stalling of the replication fork, its collapse and the generation of a DNA DSB (21). Among evaluated PARP inhibitors, olaparib, niraparib, and rucaparib are approximately 100-fold more potent than veliparib, while talazoparib has the most enhanced trapping potency (10). It has been suggested a correlation between increased PARP trapping and high myelosuppression, which results on variation in dosing among PARP inhibitors.
Apart from mutations in BRCA1/2, genomic alterations involving other genes in HR deficiency pattern have been recognized (22). The term “BRCAness” describes the phenotype shared between BRCA1/2‐mutated and non‐BRCA1/2‐mutated ovarian cancers, resulted in severe chromosomal instability due to deregulated HR (23). Indeed, BRCAness phenotype may be attributed in part to defective HR secondary to several mechanisms, including hypermethylation of the BRCA1 promoter, somatic mutations of BRCA1/2, or EMSY amplification. Furthermore, several somatic mutations in genes beyond BRCA have been recognized in a wide variety of tumors. For example, aberration of ATM, BRIP1, RAD50, RAD51C, RAD51D, RAD52, and DNA-dependent protein kinase (DNA-PK) is therapeutically important as expands the sensitivity to PARP inhibition beyond germline BRCA1/2 mutations (24). Ongoing efforts are directed towards clinical application of synthetic lethality and interaction between PARP inhibition and HR deficiency. To this end, a precise comprehension of the implications of the different PARP inhibitors is challenging.
Clinical applications of PARP inhibitors in ovarian cancer
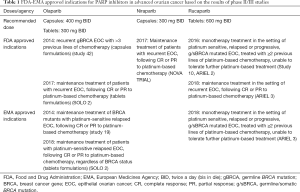
PARP inhibitors were originally developed as radio- and chemo-sensitising drugs, and are being investigated to a different extent and settings in EOC and other solid tumors (25). Table 1 depicts the PARP inhibitors, which have obtained approval by FDA, and/or EMA for the treatment of EOC. Currently, novel agents are in clinical development. Veliparib was initially demonstrated in 2007 to potentiate the preclinical activity of temozolomide, platinum agents, and radiotherapy in a variety of tumors (9). Talazoparib specifically in the treatment of EOC is still at an early stage of clinical development. However, there are studies actively recruiting patients for the evaluation of talazoparib in several solid tumors. Talazoparib has currently EMA (and FDA) approval for metastatic breast cancer.
Full table
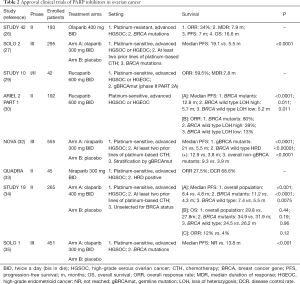
Historically, EMA approved in 2014 a capsule formulation of olaparib in maintenance setting for BRCA carriers with recurrent high grade serous EOC, or primary peritoneal cancer (study 19) (24). At the same year, FDA approved olaparib as the first-in-class PARP inhibitor for germline BRCA-mutated patients, previously treated with at least three lines of chemotherapy (study 42) (26). The tablet formulation of olaparib has been approved by both agencies as maintenance therapy for patients with platinum-sensitive relapsed EOC regardless of BRCA status (SOLO 2) (24,27). FDA approved olaparib maintenance treatment on December 19, 2018, based on the results of SOLO 1 trial (NCT01844986), examined the efficacy of olaparib versus placebo in subjects with BRCA-mutated advanced EOC, who were in complete response (CR) or partial response (PR) to first-line platinum-based chemotherapy (28).
Rucaparib has been approved by FDA and EMA in December 2016 and May 2018 respectively, for patients who have been treated with two or more prior lines of platinum-based chemotherapy, and cannot tolerate further platinum-based chemotherapy. The efficacy was based on integrated analyses of data from study 10 and ARIEL2 (29-31).
FDA and EMA approved niraparib (March and November 2017, respectively) for the maintenance treatment of the responders to platinum-based chemotherapy (NOVA trial) (32). In June 2018 were presented the results of a phase II study of niraparib in heavily pre-treated patients with recurrent ovarian cancer (Quadra Trial) (33). Registration studies that led to approvals of PARP inhibitors for treatment of EOC are resumed in Table 2.
Full table
Based on the distinct chemical structures of PARP inhibitors and various off-target effects, the therapeutic strategy of re-challenge with a PARP inhibitor following disease progression needs to be further developed. In June 2019 was presented at the American Society of Clinical Oncology (ASCO) the largest clinical trial of prospective evaluation of PARP inhibitors failure, correlating tissue genomic mechanisms of resistance (36).
Preclinical pharmacokinetics and pharmacodynamics of veliparib
The pharmacokinetic profile of veliparib is characterized by high oral bioavailability and rapid absorption. The administration of the immediate-release formulation BID (bis in die) resulted in a peak-to-trough concentration ratio of 0.45 µM. Veliparib passes through the blood–brain barrier; its combination with temozolomide is highly effective in the treatment of intracranial tumors (9). The activity of veliparib combined with temozolomide has been demonstrated across a broad histologic spectrum of models in B-cell lymphoma, lung, pancreatic, ovarian, breast, and prostate cancer xenografts (37). Veliparib is primarily excreted from tubular cells into urine via OCT2. With this regard, drug dosage adjustment should be based on the creatinine clearance, whereas concurrent treatment with OCT2-inhibitors such as cimetidine, results in higher therapeutic dose of veliparib (9).
In terms of mechanisms of action, apart from “PARP-trapping”, it is fundamental the sensitizing effect of veliparib to DNA-damaging drugs, including oxaliplatin, irinotecan, cisplatin, carboplatin and cyclophosphamide, and equally the radiotherapy (38).
Veliparib in clinical practice
The analysis of ongoing studies, assessing veliparib as single agent or, in combination with cytotoxics, revealed an overall objective response rate (ORR) ranging from 14.3% to 79%.
Phase I/II studies of veliparib monotherapy
A phase I trial, presented in 2014, assessed pharmacokinetics, pharmacodynamics and clinical efficacy of veliparib (39). Among 88 enrolled patients with platinum-refractory EOC or basal-like breast cancer, 60 were BRCA mutants. The recommended phase II dose (RP2D) was 400 mg BID, and the half-life 5.2 hours. ORR was higher in BRCA mutated, as compared to BRCA wild-type patients (23% and 4%, respectively). The most common toxicities included nausea, fatigue, and lymphopenia.
More recently, a phase I/II trial evaluated veliparib monotherapy in 48 subjects with germline BRCA mutated EOC (40). Veliparib was given BID in a 4-weekly treatment cycle, and the maximum tolerated dose (MTD) was 300 mg BID. Platinum-sensitive subset of patients attained longer PFS (P=0.037) and OS (P=0.02) than platinum-resistant. The high ORR of 65% (6% CR, 59% PR), in patients with relapsed, platinum-resistant ovarian cancer, should be highlighted. Overall, the tolerance was acceptable, and most common treatment related adverse events included grade 2 fatigue and nausea (22% each), followed by vomiting (9%).
In a small phase I dose-escalation study, 14 out of 16 enrolled Japanese patients had high-grade serous EOC and were treated with veliparib BID (41). The RP2D was 400 mg BID, whilst two patients experienced PR as best achieved response. The most prevalent grade 3 or 4 toxicities included fatigue and manifestations of the gastrointestinal system.
Based on the promising results of early phase studies, the single-arm, phase II, Gynecologic Oncology Group (GOG) 280 trial (NCT01540565) was published in 2015 (42). Veliparib was administered at a dose of 400 mg BID to a cohort of 50 BRCA mutated EOC patients, pretreated with a maximum of three lines of chemotherapy. The ORR of veliparib was 26% [90% confidence interval (CI), 16–38%], and the study met its primary endpoint. Furthermore, subgroup analysis revealed responses of 35% and 20%, in platinum-sensitive and resistant setting respectively, which was not significantly different (P=0.33). However, 31 patients (62%) experienced progressive disease while on treatment. Treatment-associated adverse events were not prominently featured; anemia and leukopenia were of grade 1/2, whilst nausea and vomiting occur mostly during first cycle treatment.
Table 3 lists the available phase I and II studies of single agent veliparib.
Phase I studies of veliparib combined with chemotherapy
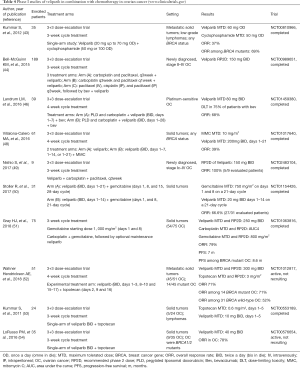
In 2012, Kummar et al. published the report of a single-arm, phase I study (NCT00810966), evaluated veliparib combined with metronomic oral cyclophosphamide (43). Thirty-five patients diagnosed with both lymphomas and refractory solid tumors, including 11 EOC, were enrolled. A standard 3+3 escalation design was employed, and starting dose of veliparib was 20 mg daily, combined with cyclophosphamide for the first 7, 14, or 21 days of the cycle. Cyclophosphamide was given 50 mg daily throughout a 3-weekly schedule. The MTD was obtained at veliparib 60 mg with cyclophosphamide 50 mg daily. As far as treatment efficacy is concerned, 7 participants (20%) experienced PRs.
In 2015 was presented the report of a phase I study of veliparib in combination with bevacizumab, paclitaxel and carboplatin in newly diagnosed patients with stage II–IV EOC or carcinosarcoma (GOG 9923; NCT00989651) (44). Veliparib starting dose was 30 mg BID given on 3-weekly cycles for the initial 6 treatment cycles. Bevacizumab was administered at 15 mg/kg intravenously, each first day 1 from cycle 2 to 22. The RP2D for veliparib was 150 mg BID in combination with the remaining regimens. Based on the NCT00989651, it is currently active the 3-arm phase III trial GOG 3005 (NCT02470585) (45).
At the same setting of the combination of veliparib with chemotherapy and bevacizumab, the phase I, GOG 9927 trial, enrolled 39 patients with relapsed platinum-sensitive EOC (NCT01459380) (46). The recommended MTD of veliparib was 80 mg BID, when was combined with pegylated liposomal doxorubicin 30 mg/m2 and carboplatin area under the curve (AUC) 5 on 4-weekly cycle. At MTD, 12 additional patients were enrolled and treated with bevacizumab. Among them, 9 exhibited dose-limiting toxicities, such as thrombocytopenia, neutropenia, hypertension, and sepsis.
It has been suggested that mitomycin C (MMC) is involved in generating DNA DSBs, activation of the Fanconi anemia (FA) pathway and veliparib-induced sensitization (47). Based on this concept was conducted a 3+3 dose escalation trial of veliparib as monotherapy, or combined with MMC. Sixty-one patients with HR deficient solid tumors were enrolled and randomized to each arm, through 14 dose levels (NCT01017640) (48). The MTD for single agent veliparib was 300 mg BID to FA-deficient patients. In the combination strategy, MMC was recommended at dose of 10 mg/m2, followed by veliparib 200 mg BID in a 4-weekly cycle with 21 days on and 7 days off. Veliparib as monotherapy did not produce a substantial number of tumor regressions. This modest clinical benefit is associated with veliparib’s spectrum of doses below MTD, and the additional antiapoptotic stimulus to which the repair deficient cell has become addicted.
In 2017 was published a small Japanese phase I dose-escalation trial (NCT02483104), evaluated in newly diagnosed advanced EOC, veliparib in combination with 3-weekly cycles of carboplatin AUC 6 and paclitaxel 80 mg/m2 (days 1, 8, 15) (49). Patients were treated with the platinum-doublet chemotherapy for six cycles in total; veliparib was incorporated throughout the course of treatment, and the RP2D was 150 mg BID. Among 5 assessed for response patients, 4 experienced PR, and 1 CR, respectively. However, these findings should be interpreted cautiously, taking into account the small sample size, and lack of random assignment and control group.
Another small phase I study (NCT01154426) evaluated veliparib combined with single agent gemcitabine in advanced solid tumors (50). Gemcitabine was given at dose of 500–750 mg/m2, administered either thrice on 4-weekly, or twice on 3-weekly schedule. Veliparib was escalated from 10 to 40 mg BID during gemcitabine’s weeks. Among 31 enrolled patients, 23 developed grade 3/4 side effects, primarily myelosuppression. The recommended MTD were 750 mg/m2 for gemcitabine and 20 mg for veliparib BID on the 3-weekly regime. Among 27 patients, 3 achieved PR and 15 stable disease (SD), respectively. However, correlation between response and BRCA status is difficult to be justified, and the combination should be further explored.
Veliparib combined with the doublet of carboplatin/gemcitabine has been investigated in a phase I dose-escalation study of 75 patients with advanced EOC and breast cancers (NCT01063816) (51). The most prevalent adverse event was the myelosuppression resulting in discontinuation in 11% of patients, and dose reduction for veliparib and gemcitabine were required in 20 (27%) and 27 patients (36%), respectively. Median PFS for the entire study population was 7.0 months (95% CI, 5.3–8.4 months). This PFS benefit was more prominent in BRCA carriers [8.6 months (95% CI, 7.1–11.7 months)] than in BRCA wild-type/unknown subgroup [5.9 months (95% CI, 4.1–9.9 months)]. Equally, BRCA-mutants achieved higher ORR of 68.9% as compared to the 42.8% of the wild-type/unknown BRCA patients.
Finally, a phase I study (NCT01012817) evaluated the combination of veliparib and weekly topotecan in several solid tumors, including EOC (52). The treatment was well tolerated, and in line with the previous studies, resulted in prolonged ORR in BRCA1/2, or RAD51D mutants.
Table 4 details phase I studies of veliparib combined with chemotherapy.
Full table
Phase II/III studies of veliparib combined with chemotherapy
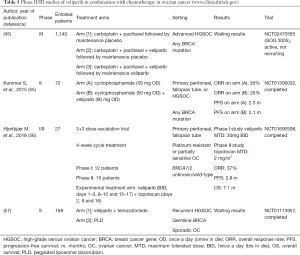
A randomized phase II trial (NCT01306032) randomized 72 pretreated, BRCA-mutant, EOC patients to the combination of veliparib with low dose cyclophosphamide, versus cyclophosphamide monotherapy (55). DNA repair defects were not predictive biomarkers for either cyclophosphamide single agent or the veliparib combination. Finally, neither ORR (11.8% versus 19.4%, respectively), nor median PFS (2.1 versus 2.3 months, respectively; P=0.68) were improved with the combination. Based on that, the trial was early terminated.
Taken the in vitro synergy of topotecan with veliparib, a phase I/II dose escalation clinical trial was conducted to investigate the combination in the setting of recurrent, BRCA1/2 wild-type or unknown EOC (NCT01690598) (56). Twenty-seven enrolled patients were treated with an initial dose of veliparib, 30 mg BID, and topotecan, 3 mg/m2 in 4-week treatment cycles. The reported efficacy was modest with median PFS of 2.8 months (95% CI, 2.6–3.6 months), and OS of 7.1 months (95% CI, 4.8–10.8 months). However, these findings should be interpreted in light of the negative prognostic factors of the study population. Haematological toxicities of grade 1 and 2 included mostly anemia (81.5%), followed by thrombocytopenia (29.6%) and neutropenia (22.2%).
Furthermore, the results of a randomized phase II study in recurrent high-grade serous EOC, evaluating veliparib combined with temozolomide versus pegylated liposomal doxorubicin are pending (NCT01113957) (57). Finally, the phase III GOG 3005 is an ongoing, randomized, double-blind trial, with aim to investigate the efficacy of veliparib in combination with carboplatin and paclitaxel in high-grade serous EOC, or primary peritoneal cancer patients (NCT02470585) (45). The recruitment target size is 1,140 patients, and this is the only phase III trial of veliparib in first-line treatment.
Phase II/III clinical trials of veliparib in combination with chemotherapy for the treatment of EOC are resumed in Table 5.
Full table
Veliparib in combination with radiotherapy
Preclinical evidence suggests that low-dose fractionated whole abdominal radiation (LDFWAR) combined with veliparib is an effective therapeutic option. A phase I dose escalation trial enrolled 22 patients with advanced solid tumors and peritoneal carcinomatosis, including 8 subjects with EOC (58). SD was maintained for 24 weeks or longer in 33% of participants. PFS was 7.92 months in the platinum-sensitive setting versus 3.58 months in the platinum-resistant subset.
In the final publication, 32 patients were finally enrolled, including 18 with EOC (56%) (59). The established MTD and RP2D for veliparib was 250 mg BID. Patients with platinum-resistant and those with platinum-sensitive recurrence, achieved a median OS of 5.8 and 10.9 months, respectively. The most common haematological adverse event of grade 3/4 was lymphopenia (59%), followed by thrombocytopenia (12%), anemia (9%), and neutropenia (6%). However, due to lack of specific biomarkers, incorporation of somatic genomic testing and HR deficiency score should be planned, in view of optimization of the efficacy of this therapeutic strategy.
Early randomized studies of veliparib in combination with radiotherapy are depicted in Table 6.
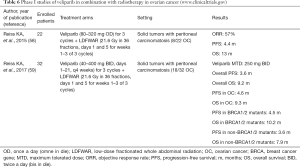
Full table
Talazoparib
Talazoparib in the treatment of EOC is still at an early stage of clinical development. However, preclinical studies have demonstrated activity in several solid tumors (60-63). Following olaparib, talazoparib was the second FDA and EMA approved drug for BRCA-mutated, HER2-negative breast cancer. Superior radiosensitizing capacity of talazoparib as compared to veliparib is probably based to its enhanced PARP trapping ability (64). Talazoparib has been shown to be the more potent PARP inhibitor (10), but equally has the highest rates of myelosuppression, particularly anemia and neutropenia in clinical trials (65).
Phase I studies of talazoparib monotherapy
Talazoparib was initially evaluated in 2017, with the first-in-human, 2-stage, dose-escalation, phase I study in over 100 patients with germline BRCA1/2 mutated advanced or recurrent solid tumors, previously treated with platinum-based chemotherapy (NCT01286987) (66). Thirty-four ovarian cancer patients were enrolled in 9 cohorts, and the established MTD in the expansion cohort was 1 mg per day. A subset of 17 patients with BRCA1/2-mutant high-grade serous EOC was treated with doses of at least 0.1 mg/day. Radiological, biochemical and clinical benefit responses were achieved by 44%, 70% and 82%, respectively; nevertheless, response rates were much lower in patients with platinum-resistant disease (20%). The estimated median PFS was 36.4 weeks, whilst the achieved ORR to talazoparib was 50% (7/14) in BRCA1/2 patients. Treatment related adverse events included mostly fatigue (37%), anemia (35%), and nausea (32%), whilst grade 3 to 4 side effects were anemia (24%) and thrombocytopenia (18%).
At the same period was published a phase I/II trial evaluated talazoparib combined with carboplatin in several solid tumors, including EOC (8%) (67). Twenty-four patients were enrolled in four cohorts. Frequent grade 3/4 side effects were neutropenia (63%), which was more prominent in germline BRCA mutants, followed by anemia (38%), thrombocytopenia (29%), and fatigue (13%). One complete and two PRs (14%) were achieved by patients with germline BRCA1/2 mutations. Finally, POSITION is an ongoing phase I study assessing the influence of talazoparib on DNA copy number and RNA expression in patients with advanced stage EOC (NCT02316834) (68).
Table 7 provides summary results of phase I studies of talazoparib for treatment of ovarian cancer.
Phase II/III studies of talazoparib monotherapy beyond ovarian cancer
Currently, there are not available phase II or III clinical trials of talazoparib monotherapy in EOC. However, such data could be extrapolated from ongoing studies in metastatic breast cancer (65,69). Indeed, the benefit of talazoparib, specifically in BRCA mutants, has been reported in the phase II ABRAZO study (NCT02034916) (69). Eighty-four patients, pre-treated with platinum or other cytotoxic regimens, were enrolled in the study. The reported ORR for those with BRCA1/2 mutations was 23% and 33%, respectively. Similarly, triple-negative breast cancer patients, and those with expressed estrogen and progesterone receptors, achieved an ORR of 26% and 29%, respectively.
FDA and EMA granted standard approval of talazoparib in advanced, HER2-negative advanced or metastatic breast cancer with germline BRCA1/2 mutations, based on data gathered from EMBRACA study (NCT01945775) (65). This is a phase III, open-label study, which compared talazoparib with standard single agent treatment. The primary endpoint of median PFS was 8.6 months in talazoparib arm, significantly higher than 5.6 months in the chemotherapy arm [HR: 0.54 (95% CI, 0.41–0.71), P<0.0001]. Furthermore, response rates in talazoparib and chemotherapy group, were 63% and 27%, respectively. Similarly, quality of life was importantly improved in favour of talazoparib. Efficacy of the agent in triple-negative, BRCA wild-type breast cancer, will be evaluated by the ongoing phase II trial NCT02401347.
Several studies are in progress in prostate cancer. NCT03148795 is a phase II study aiming to assess talazoparib in patients with metastatic, castration resistant disease with defects in DNA repair mechanisms (70), whilst phase III study TALAPRO-2 (NCT03395197), is evaluating the addition of talazoparib to enzalutamide at the same setting (71).
Additionally, the single-arm phase 2 study NCT01989546 is still recruiting patients with several solid tumors and BRCA1/2-mutations, for the evaluation of talazoparib in platinum-sensitive setting (72).
As far as concerned ovarian cancer, two phase II trials have already been withdrawn (Table 8). NCT02326844 enrolled patients with BRCA1/2 mutations, following primary progression on prior PARP inhibitor therapy (73). This study addresses the important issue of whether re-challenging with an alternative PARP inhibitor may be associated with therapeutic benefit. Similarly, the withdrawn phase II randomized study NCT02836028 had been planned to assess talazoparib combined or not with temozolomide in patients with relapsed ovarian cancer and defects in DNA repair pathway (74).
Conclusions and future directions
PARP inhibitors have attracted great attention and illustrate a paradigm of bench-to-bedside medicine. HR deficiency remains a strong predictor of clinical benefit from these agents. Besides ovarian cancer, PARP inhibitors may be effective in subsets of patients with breast, prostate, and even pancreatic tumors. On December 27, 2019 FDA approved olaparib for the maintenance treatment of patients with metastatic pancreatic cancer, who were carriers of germline BRCA1/2 mutations, based on the results of POLO trial (NCT02184195). The ORR was 23.1% in the olaparib versus 11.5% in the placebo arm, whereas median duration of response was 24.9 months as compared to 3.7 months, respectively. Mutations in DNA repair related genes are frequent in those tumors, which highlights further that evaluation of molecular alterations should be incorporated in clinical practice. Apparently, combination treatment strategies can induce HR pathway deficiency in cancers with de novo or acquired HR proficiency to PARP inhibitors. Moreover, PARP inhibitors may be effective in patients with somatic BRCA1/2 mutations to the same extent as in those with germline BRCA1/2 mutations. As such, somatic genomic analysis and clinical qualification of biomarkers, enabling patient stratification, promote delivery of precision medicine. Adverse events associated with PARP inhibitors should be carefully evaluated. Myelosuppression may require dose reduction. Optimization of toxicities could be achieved by modifying treatment modalities (continuous versus intermittent, concurrent to chemotherapy versus maintenance). Several clinical trials are ongoing, in different settings. Even though newer PARP inhibitors, demonstrate increased potency, it has not yet been fully clarified whether this translates into greater efficacy.
Acknowledgments
Funding: The authors acknowledge support from the Research and Innovation department of Medway NHS Foundation Trust.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stergios Boussios and Nicholas Pavlidis) for the series “Ovarian Cancer: State of the Art and Perspectives of Clinical Research” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.156). The series “Ovarian Cancer: State of the Art and Perspectives of Clinical Research” was commissioned by the editorial office without any funding or sponsorship. SB and NP served as the unpaid Guest Editors of the series. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Boussios S, Karathanasi A, Cooke D, et al. PARP Inhibitors in Ovarian Cancer: The Route to "Ithaca". Diagnostics (Basel) 2019;9:55. [Crossref] [PubMed]
- Papa A, Caruso D, Strudel M, et al. Update on Poly-ADP-ribose polymerase inhibition for ovarian cancer treatment. J Transl Med 2016;14:267. [Crossref] [PubMed]
- King MC, Marks JH, Mandell JB, et al. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003;302:643-6. [Crossref] [PubMed]
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913-7. [Crossref] [PubMed]
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917-21. [Crossref] [PubMed]
- Venkitaraman AR. A growing network of cancer-susceptibility genes. N Engl J Med 2003;348:1917-9. [Crossref] [PubMed]
- Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer 2004;4:814-9. [Crossref] [PubMed]
- Boussios S, Karihtala P, Moschetta M, et al. Veliparib in ovarian cancer: a new synthetically lethal therapeutic approach. Invest New Drugs 2020;38:181-93. [Crossref] [PubMed]
- Shen Y, Rehman FL, Feng Y, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res 2013;19:5003-15. [Crossref] [PubMed]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature 1993;362:709-15. [Crossref] [PubMed]
- Kraus WL. PARPs and ADP-Ribosylation: 50 Years … and Counting. Mol Cell 2015;58:902-10. [Crossref] [PubMed]
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 2001;411:366-74. [Crossref] [PubMed]
- Aymard F, Bugler B, Schmidt CK, et al. Transcriptionally active chromatin recruits homologous recombination at DNA double-strand breaks. Nat Struct Mol Biol 2014;21:366-74. [Crossref] [PubMed]
- Radhakrishnan SK, Jette N, Lees-Miller SP. Non-homologous end joining: emerging themes and unanswered questions. DNA Repair (Amst) 2014;17:2-8. [Crossref] [PubMed]
- Plummer R. Perspective on the pipeline of drugs being developed with modulation of DNA damage as a target. Clin Cancer Res 2010;16:4527-31. [Crossref] [PubMed]
- Nieborowska-Skorska M, Sullivan K, Dasgupta Y, et al. Gene expression and mutation-guided synthetic lethality eradicates proliferating and quiescent leukemia cells. J Clin Invest 2017;127:2392-406. [Crossref] [PubMed]
- Langelier MF, Riccio AA, Pascal JM. PARP-2 and PARP-3 are selectively activated by 5' phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1. Nucleic Acids Res 2014;42:7762-75. [Crossref] [PubMed]
- Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017;355:1152-8. [Crossref] [PubMed]
- Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet 2008;9:619-31. [Crossref] [PubMed]
- O'Neil NJ, Bailey ML, Hieter P. Synthetic lethality and cancer. Nat Rev Genet 2017;18:613-23. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609-15. Erratum in: Nature. 2012 Oct 11;490(7419):298. [Crossref] [PubMed]
- Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer 2016;16:110-20. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852-61. [Crossref] [PubMed]
- Boussios S, Karihtala P, Moschetta M, et al. Combined Strategies with Poly (ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer: A Literature Review. Diagnostics (Basel) 2019;9:87. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274-84. [Crossref] [PubMed]
- Olaparib Maintenance Monotherapy in Patients with BRCA Mutated Ovarian Cancer Following First Line Platinum Based Chemotherapy. (SOLO-1) [(accessed on 24 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01844986
- Kristeleit R, Shapiro GI, Burris HA, et al. A Phase I-II Study of the Oral PARP Inhibitor Rucaparib in Patients with Germline BRCA1/2-Mutated Ovarian Carcinoma or Other Solid Tumors. Clin Cancer Res 2017;23:4095-106. [Crossref] [PubMed]
- Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017;18:75-87. [Crossref] [PubMed]
- Oza AM, Tinker AV, Oaknin A, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol 2017;147:267-75. [Crossref] [PubMed]
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375:2154-64. [Crossref] [PubMed]
- Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:636-48. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382-92. [Crossref] [PubMed]
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018;379:2495-505. [Crossref] [PubMed]
- Lheureux S, Oaknin A, Swati Garg S, et al. Evolve: A post PARP inhibitor clinical translational phase II trial of cediranib-olaparib in ovarian cancer—A Princess Margaret Consortium – GCIG Phase II Trial. J Clin Oncol 2019;37:5521. [Crossref]
- Palma JP, Wang YC, Rodriguez LE, et al. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res 2009;15:7277-90. [Crossref] [PubMed]
- Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 2007;13:2728-37. [Crossref] [PubMed]
- Puhalla S, Beumer JH, Pahuja S, et al. Final results of a phase 1 study of single-agent veliparib (V) in patients (pts) with either BRCA1/2-mutated cancer (BRCA+), platinum-refractory ovarian, or basal-like breast cancer (BRCA-wt). J Clin Oncol 2014;32:2570. [Crossref]
- Steffensen KD, Adimi P, Jakobsen A. Veliparib Monotherapy to Patients With BRCA Germ Line Mutation and Platinum-Resistant or Partially Platinum-Sensitive Relapse of Epithelial Ovarian Cancer: A Phase I/II Study. Int J Gynecol Cancer 2017;27:1842-9. [Crossref] [PubMed]
- Nishikawa T, Matsumoto K, Tamura K, et al. Phase 1 dose-escalation study of single-agent veliparib in Japanese patients with advanced solid tumors. Cancer Sci 2017;108:1834-42. [Crossref] [PubMed]
- Coleman RL, Sill MW, Bell-McGuinn K, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015;137:386-91. [Crossref] [PubMed]
- Kummar S, Ji J, Morgan R, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res 2012;18:1726-34. [Crossref] [PubMed]
- Bell-McGuinn KM, Brady WE, Schilder RJ, et al. A phase I study of continuous veliparib in combination with IV carboplatin/paclitaxel or IV/IP paclitaxel/cisplatin and bevacizumab in newly diagnosed patientswith previously untreated epithelial ovarian, fallopian tube, or primary peritoneal cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol 2015;33:5507. [Crossref]
- Veliparib With Carboplatin and Paclitaxel and as Continuation Maintenance Therapy in Subjects with Newly Diagnosed Stage III or IV, High-grade Serous, Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. (accessed on 24 January 2020); Available online: https://Clinicaltrials.gov/ct2/show/CT02470585
- Landrum LM, Brady WE, Armstrong DK, et al. A phase I trial of pegylated liposomal doxorubicin (PLD), carboplatin, bevacizumab and veliparib in recurrent, platinum-sensitive ovarian, primary peritoneal, and fallopian tube cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2016;140:204-9. [Crossref] [PubMed]
- Duan W, Gao L, Aguila B, et al. Fanconi anemia repair pathway dysfunction, a potential therapeutic target in lung cancer. Front Oncol 2014;4:368. [Crossref] [PubMed]
- Villalona-Calero MA, Duan W, Zhao W, et al. Veliparib Alone or in Combination with Mitomycin C in Patients with Solid Tumors With Functional Deficiency in Homologous Recombination Repair. J Natl Cancer Inst 2016;108:djv437. [Crossref] [PubMed]
- Nishio S, Takekuma M, Takeuchi S, et al. Phase 1 study of veliparib with carboplatin and weekly paclitaxel in Japanese patients with newly diagnosed ovarian cancer. Cancer Sci 2017;108:2213-20. [Crossref] [PubMed]
- Stoller R, Schmitz JC, Ding F, et al. Phase I study of veliparib in combination with gemcitabine. Cancer Chemother Pharmacol 2017;80:631-43. [Crossref] [PubMed]
- Gray HJ, Bell-McGuinn K, Fleming GF, et al. Phase I combination study of the PARP inhibitor veliparib plus carboplatin and gemcitabine in patients with advanced ovarian cancer and other solid malignancies. Gynecol Oncol 2018;148:507-14. [Crossref] [PubMed]
- Wahner Hendrickson AE, Menefee ME, Hartmann LC, et al. A phase I clinical trial of the poly(ADPribose) polymerase inhibitor Veliparib and weekly Topotecan in patients with solid tumors. Clin Cancer Res 2018;24:744-52. [Crossref] [PubMed]
- Kummar S, Chen A, Ji J, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res 2011;71:5626-34. [Crossref] [PubMed]
- LoRusso PM, Li J, Burger A, et al. Phase I Safety, Pharmacokinetic, and Pharmacodynamic Study of the Poly(ADP-ribose) Polymerase (PARP) Inhibitor Veliparib (ABT-888) in Combination with Irinotecan in Patients with Advanced Solid Tumors. Clin Cancer Res 2016;22:3227-37. [Crossref] [PubMed]
- Kummar S, Oza AM, Fleming GF, et al. Randomized Trial of Oral Cyclophosphamide and Veliparib in High-Grade Serous Ovarian, Primary Peritoneal, or Fallopian Tube Cancers, or BRCA-Mutant Ovarian Cancer. Clin Cancer Res 2015;21:1574-82. [Crossref] [PubMed]
- Hjortkjær M, Kanstrup H, Jakobsen A, et al. Veliparib and topotecan for patients with platinum-resistant or partially platinum-sensitive relapse of epithelial ovarian cancer with BRCA negative or unknown BRCA status. Cancer Treat Res Commun 2018;14:7-12. [Crossref] [PubMed]
- A Trial of ABT-888 in Combination with Temozolomide Versus Pegylated Liposomal Doxorubicin Alone in Ovarian Cancer. (accessed on 24 January 2020); Available online: https://Clinicaltrials.gov/ct2/show/NCT01113957
- Reiss KA, Herman JM, Zahurak M, et al. A Phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. A Phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin Cancer Res 2015;21:68-76. [Crossref] [PubMed]
- Reiss KA, Herman JM, Armstrong D, et al. A final report of a phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy (LDFWAR) in patients with advanced solid malignancies and peritoneal carcinomatosis with a dose escalation in ovarian and fallopian tube cancers. Gynecol Oncol 2017;144:486-90. [Crossref] [PubMed]
- Engert F, Kovac M, Baumhoer D, et al. Osteosarcoma cells with genetic signatures of BRCAness are susceptible to the PARP inhibitor talazoparib alone or in combination with chemotherapeutics. Oncotarget 2017;8:48794-806. [Crossref] [PubMed]
- Herriott A, Tudhope SJ, Junge G, et al. PARP1 expression, activity and ex vivo sensitivity to the PARP inhibitor, talazoparib (BMN 673), in chronic lymphocytic leukaemia. Oncotarget 2015;6:43978-91. [Crossref] [PubMed]
- Pulliam N, Taverna P, Lyons J, et al. Novel combination therapy of DNMT inhibitor SGI-110 and PARP inhibitor BMN-673 (talazoparib) for BRCA-proficient ovarian cancer. Can Res 2015;75:2943.
- Wilkerson PM, Dedes KJ, Samartzis EP, et al. Preclinical evaluation of the PARP inhibitor BMN-673 for the treatment of ovarian clear cell cancer. Oncotarget 2017;8:6057-66. [Crossref] [PubMed]
- Laird JH, Lok BH, Ma J, et al. Talazoparib Is a Potent Radiosensitizer in Small Cell Lung Cancer Cell Lines and Xenografts. Clin Cancer Res 2018;24:5143-52. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- de Bono J, Ramanathan RK, Mina L, et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov 2017;7:620-9. [Crossref] [PubMed]
- Dhawan MS, Bartelink IH, Aggarwal RR, et al. Differential Toxicity in Patients with and without DNA Repair Mutations: Phase I Study of Carboplatin and Talazoparib in Advanced Solid Tumors. Clin Cancer Res 2017;23:6400-10. [Crossref] [PubMed]
- Talazoparib in Determining Genetic Effects on Disease Response in Patients With Advanced Ovarian, Fallopian Tube, or Primary Peritoneal Cancer (POSITION) [(accessed on 24 January 2020)]. Available online: https://clinicaltrials.gov/ct2/show/NCT02316834
- Turner NC, Telli ML, Rugo HS, et al. A Phase II Study of Talazoparib after Platinum or Cytotoxic Nonplatinum Regimens in Patients with Advanced Breast Cancer and Germline BRCA1/2 Mutations (ABRAZO). Clin Cancer Res 2019;25:2717-24. [Crossref] [PubMed]
- A Study of Talazoparib in Men With DNA Repair Defects and Metastatic Castration-Resistant Prostate Cancer [(accessed on 24 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03148795
- Talazoparib + Enzalutamide vs. Enzalutamide Monotherapy in mCRPC (TALAPRO-2) [(accessed on 24 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03395197
- Pilot Trial of BMN 673, an Oral PARP Inhibitor, in Patients With Advanced Solid Tumors and Deleterious BRCA Mutations [(accessed on 24 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01989546
- BMN 673 (Talazoparib), an Oral PARP Inhibitor, in People With Deleterious BRCA1/2 Mutation-Associated Ovarian Cancer Who Have Had Prior PARP Inhibitor Treatment [(accessed on 24 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02326844
- A Study Evaluating Talazoparib in Relapsed Ovarian, Fallopian Tube, and Peritoneal Cancer [(accessed on 24 January 2020)]. Available online: https://clinicaltrials.gov/ct2/show/NCT02836028





