
Does the conventional dosage of linezolid necessitate therapeutic drug monitoring?—Experience from a prospective observational study
Introduction
Linezolid, the first commercially available oxazolidinone antibiotic, is a synthetic antibiotic that inhibits the growth of a variety of Gram-positive bacteria such as Streptococci, Staphylococci, and Enterococci by preventing bacterial protein synthesis (1). It also has strong antimicrobial activity against resistant Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) (2). It has been approved for the treatment of infections including bacterial pneumonia, skin and skin structure infections, and VRE infections (3).
The pharmacokinetics of linezolid in healthy subjects has been well studied. Linezolid is well absorbed after oral administration with a bioavailability of 100% (4). The volume of distribution approximates 0.5–0.8 L/kg, indicating excellent penetration in almost all tissues (5). Linezolid is metabolized by oxidation of the morphine ring to inactive metabolites including PNU-142586 and PNU-14230 in a non-enzymatic process (6). The clearance rate is 80±29 mL/min with approximately one-third of the linezolid eliminating via a renal route and the remaining eliminating through non-renal clearance (4,7). The elimination of the half-life of linezolid is about 5–7 h. Based on those intrinsic pharmacokinetics characteristics, a fixed standard dosing of linezolid at 600 mg every 12 h is recommended for all adult patients (8,9). Moreover, it has been assumed that the plasma exposure of linezolid remains similar in different categories of patients, thus dose adjustment and therapeutic drug monitoring (TDM) are unnecessary in most cases.
However, recent studies have noticed a high variability of linezolid plasma concentrations in critically ill patients treated with this standard regimen (10-13). Extensive evidence is now available showing significant inadequate plasma concentrations with increased risk of treatment failure in patients (14,15). Conversely, high linezolid trough plasma concentrations with risk of thrombocytopenia has been documented in some patients (16). Therefore, routine TDM of linezolid has been proposed for certain occasions to prevent therapeutic failure and adverse events.
The purposes of this prospective observational study were to define the desired range of linezolid Cmin, to assess the interindividual variability in linezolid serum exposure, to identify the prevalence of attainment of the desired range of linezolid exposure, and to define if TDM of linezolid may be necessary in some settings for the Chinese population.
Methods
Study design
This prospective observational study was carried out between January 2019 and October 2019 at Ruijin Hospital, Shanghai, China. Adult patients treated with oral and/or intravenous linezolid at the standard dosage of 600 mg every 12 h for suspected or documented multidrug-resistant (MDR) Gram-positive bacterial infections were included in this study. Patients with hematological disease or malignant cancer were excluded.
This study was approved by the regional ethical committee.
Measurement of linezolid serum concentrations
Venous blood samples were drawn 30 min before the next administration to assess the Cmin of linezolid. Linezolid serum concentrations were tested by means of the high-performance liquid chromatography (HPLC) method. Chloramphenicol was used as the internal standard. The separation was performed on a Symmetry C18 Column (4.6 mm × 150 mm, 5 µm) at 30 °C. The mobile phase consisted of acetonitrile-water (28:72) with a flow rate of 1.0 mL/min. The ultraviolet (UV) detection wave length was 254 nm. The calibration curves of linezolid showed good linear regression in the range of 0.25–50 mg (r2=0.9997). The limit of detection was 0.05 mg/L. The mean absolute recovery was 95.68%, and the method recovery was 100–102%. Intra and inter-day variations were less than 6%.
Estimation of creatinine clearance
Creatinine clearance (CrCL) was estimated by the Cockcroft and Gault formula.
Toxicity analysis
Thrombocytopenia was defined as a platelet count of <100×103 cells/µL at any time during linezolid treatment for patients with platelet at or above the lower limit of normal (100×103 cells/µL) at baseline. For patients with lower platelet counts at baseline (<100×103 cells/µL), thrombocytopenia was defined as a 30% reduction from baseline.
Desired therapeutic range
Cmin values of >2 mg/L were used for efficacy evaluation since linezolid trough concentration has been linearly correlated with estimated area under the curve (AUC)24 (12), and Cmin value of >2 mg/L has been identified as a predictor of >80% probability of bacterial eradication (17,18). Cmin values of <2 mg/L were defined as underexposure. A logistic regression model was used to estimate the correlation between linezolid Cmin and the probability of thrombocytopenia. The higher limit of normal linezolid plasma exposure was defined as Cmin value associated with ≥50% odds of thrombocytopenia. Cmin values higher than the upper limit of normal linezolid exposure were defined as overexposure.
Statistical analysis
The Kolmogorov-Smirnov test was conducted to assess if the data were normally distributed. Descriptive data were expressed as medians and interquartile ranges (IQR) or means ± standard deviations (SD) accordingly. Continuous variables were compared by the Student’s t-test. A P value of <0.05 was considered to achieve statistical significance. The statistical analysis was conducted with the SPSS statistical package (version 23.0, SPSS Inc., Chicago, IL, USA).
Results
A total of 84 patients who had 153 Cmin assessed during treatment with a conventional dosage of linezolid were included in the study. Detailed patient characteristics are shown in Table 1. Mean age was 69.6 years. The majority of patients were male (57/27, 67.9%). Median estimated CrCLC-G and serum albumin were 77.4 mL/min and 11.9 g/dL respectively.
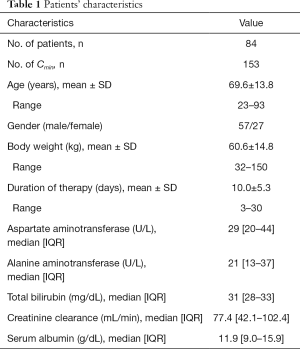
Full table
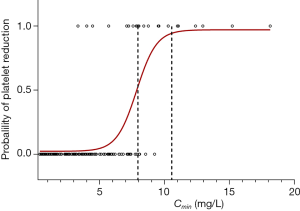
Thrombocytopenia appeared in 21.43% (18/84) of patients treated with conventional dosing of linezolid. A logistic regression model showed significant correlation between the probability of thrombocytopenia and linezolid Cmin (Figure 1). The estimated probability of thrombocytopenia was 50% in the presence of a Cmin of 7.85 mg/L and rose to 95% in the presence of a Cmin of 10.55 mg/L.
Median linezolid trough concentration was 3.43 mg/L (IQR 1.59–5.93) (Figure 2). Among all samples, only 57.52% (88/153) fell within the desired range of linezolid Cmin (2–8 mg/L), 31.37% (48/153) had trough concentrations lower than 2 mg/L, and overexposure occurred in 11.11% (17/153) of the patients.
Beeswarm plots of linezolid Cmin showed similar distributions between the oral and the intravenous route for the administration of linezolid (median 3.61 vs. 3.41 mg/L, P=0.09) (Figure 3). No significant linear relationship between age (r2=0.007) and Cmin was observed. There was also no significant difference of linezolid distribution between males and females (median 3.46 vs. 3.30 mg/L, P=0.80). Furthermore, no significant linear relationships between either body weight (r2=0.06) (Figure 4) or estimated CrCLC-G (r2=0.11) (Figure 5) and Cmin were detected. Univariate analyses of weight or CrCLC-G for association with the risk of linezolid underexposure and overexposure were conducted (Tables 2,3). Results showed that weight was not associated with either linezolid underexposure or overexposure. Estimated CrCLC-G ≥100 mL/min was significantly associated with the risk of linezolid underexposure (OR 4.121; 95% CI, 1.945–8.731; P<0.001). Estimated CrCLC-G ≤40 mL/min was significantly associated with the risk of linezolid overexposure (OR 3.761; 95% CI, 1.324–10.681; P=0.013).

Full table

Full table
Discussion
To the best of our knowledge, this is the largest prospective observational study of linezolid TDM conducted in Chinese population.
Linezolid is an oxazolidinone antibiotic with a bioavailability of almost 100% (3). The metabolism of linezolid mainly involves a non-enzymatic pathway (19). Non-renal clearance accounts for 65% of linezolid clearance (19). Based on its physicochemical characteristics, it has been assumed that the plasma exposure of linezolid remains similar across different categories of patients, and the recommended dosage of linezolid is a fixed standard regimen of 600 mg every 12 h for all adult patients (1,8).
Our study found a high variability of linezolid plasma concentrations in patients treated with conventional dosage of linezolid at 600 mg every 12 h. Although median linezolid trough concentration in this study was similar to that observed in healthy volunteers (7,20), the range of Cmin was significantly wider (0.40–18.13 mg/L). Several recent studies have also identified great variability in linezolid exposure. One study that aimed to summarize the pharmacokinetics of linezolid in critically ill patients found significant changes of protein-binding, volume distribution, and metabolism of linezolid (21), which may be accounted for by the high inter-individual variability of linezolid exposure. Another retrospective observational study carried out in 92 patients receiving a standard dose of linezolid showed that the ranges of Cmin, Cmax, and AUC24 were significantly higher than those observed in healthy subjects (12). Other studies have also suggested that linezolid plasma exposure may vary greatly during treatment with conventional doses (10,11). The great variability observed in linezolid exposure suggests that some patients treated with this standard regimen may be at risk of underexposure or overexposure. From this perspective, it would be of interest to clarify the desired therapeutic range of linezolid in patients.
Linezolid is a time-dependent antibiotic with a minimal to modest post-antibiotic effect (22). In vivo studies have identified AUC/MIC and fT > MIC as the best predictors of efficacy (22). Higher success rates were achieved when T > MIC exceeded 85% and the AUC/MIC ratio was between 80 and 120 (23). Several studies (12,24) have identified a superior linear correlation between Cmin of linezolid and AUC24. The linear relationship between Cmin and AUC24 suggests that Cmin could be used as a predictor of linezolid exposure.
Pea et al. (12) defined the efficacy threshold for linezolid as Cmin of ≥2 mg/L due to the notion that the MIC90 for linezolid against both MR staphylococci and VR enterococci is 2 mg/L. Cmin value of >2 mg/L has been identified as a predictor of >80% probability of bacterial eradication in other studies as well (17,18). A logistic regression model was used to estimate the correlation between linezolid Cmin and the probability of thrombocytopenia in our study. The results showed that the estimated probability of thrombocytopenia was 50% in the presence of Cmin of 7.85 mg/L. Therefore, the desired therapeutic range of linezolid was defined as 2–8 mg/L based on the experience from this study. In the past few years, other studies have provided further evidence in supporting the reliability of this range. The desired range of linezolid Cmin was defined as 2 to 7 mg/L to obtain efficacy and to prevent dose-dependent adverse effects (25). Dong et al. (18) also found that Cmin ≥2 mg/L was associated with clinical efficacy, while Cmin >6.3 mg/L could result in a probability more than 50% of thrombocytopenia. Likewise, a toxicodynamic model showed that linezolid Cmin of 8.06 mg/L may result in thrombocytopenia by 50% (26).
Of note, only 57.52% fell within the desired range of linezolid Cmin in the present study. Significant underexposure with increased risk of treatment failure occurred in 31.37% of the patients, while overexposure with increased toxicity risk was observed in 11.11% of the patients. This phenomenon indicates that TDM might be valuable for linezolid. Recently, several studies further supported the necessity of TDM for linezolid in some categories of patients (12,18,25). Moreover, it would be of importance to clarify the factors which might account for this variability.
According to the drug specification, mean trough concentration is 6.15±2.94 mg/L for oral linezolid at a dosage of 600 mg every 12 h, while mean trough concentration for intravenous linezolid at standard dosing is 3.68±2.36 mg/L. The results of this study showed similar median trough concentrations between the oral and the intravenous route for the administration of linezolid (median 3.61 vs. 3.41 mg/L, P=0.09). We indeed found a difference in the desired range achieved rate between oral (60.45%) and intravenous (31.58%) linezolid. However, it is important that we do not jump to the conclusion that lower proportion of desired therapeutic range was attained for intravenous linezolid due to the significant difference in the sample sizes between oral and intravenous linezolid (134 vs.19). Future research is needed to clarify the issue by expanding the samples' quantity.
There was no significant difference of linezolid distribution found between males and females in this study. No significant linear relationships between either body weight or age and Cmin were detected. This is in agreement with findings of Pea et al. (11) who reported that the distribution of linezolid trough concentration was not associated with body-mass index (BMI) and/or total body weight (TBW), implying that dose adjustment based on body weight alone is not necessary. Bhalodi et al. also found that linezolid Cmin in obese volunteers was similar to that of non-obese patients (27). However, underexposure to linezolid was observed among obese patients in other studies (12,15,28,29). Based on the above-mentioned conflicting results, we assume that linezolid exposure may be associated with the degree of obesity. No definite conclusions can be drawn based on the limited numbers of this study.
No significant linear relationship between estimated CrCLC-G and linezolid trough concentration was detected in this study. Univariate analyses of CrCLC-G for association with linezolid exposure found that estimated CrCLC-G ≥100 mL/min was significantly associated with the risk of linezolid underexposure, while estimated CrCLC-G ≤40 mL/min was significantly associated with the risk of linezolid overexposure, implying that CrCLC-G was a factor that might independently predict the risk of inappropriate linezolid exposure. This is in agreement with findings from other studies (11,29,30).
Drug-drug interactions also contribute to the extreme inter-individual variability of linezolid exposure. Linezolid is a substrate of P-gp. Induction or inhibition of P-gp has been assumed to be mechanism of drug-drug interaction. Concomitant application with potent inhibitors of P-gp such as omeprazole, amiodarone, amlodipine has been identified as a risk factor for linezolid overexposure (12). In a case report, cotreatment with clarithromycin resulted in significant higher linezolid concentrations (31). Conversely, it has been reported that co-administration with P-gp inducers [i.e., rifampin (14,32-34), venlafaxine (35), and levothyroxine (36)] could result in exceedingly lower linezolid levels. In our study, linezolid underexposures were observed in some patients who were co-administered with levothyroxine, although the limited sample size in this study could not yield a definite conclusion.
We are aware that our study has several limitations, including its observational nature, the lack of data on clinical efficacy, and the limited sample size, and thus we cannot draw definite conclusions from these findings. However, we indeed observed wide inter-individual variability of linezolid exposure in critically ill patients, which supports the fact that TDM of trough concentrations might be a useful approach to predict linezolid efficacy and toxicity. The desired range was confirmed to be 2–8 mg/L for the Chinese population in our study. We plan to expand the sample size of this study to further clarify some critical issues about TDM of linezolid in the future.
In conclusion, our study suggests that the desired range of linezolid for the Chinese population is 2–8 mg/L. Linezolid plasma concentrations may vary widely in adult patients receiving standard dosage of linezolid at 600 mg q12h. This variability is partly due to the variability in renal function, but other factors such as TBW and/or drug-drug interactions might also contribute to this. TDM may be valuable in helping to avoid the risk of treatment failure and dose-dependent toxicity. Further work is required to develop a guideline of dose adjustment in specific populations.
Acknowledgments
Funding: This study was funded by the scientific research program of Shanghai Municipal Health Commission (No. 201940197).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform (available at http://dx.doi.org/10.21037/atm.2020.03.207). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the regional ethical committee of Ruijin Hospital. Informed consent was waived due to the observational of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Batts DH. Linezolid--a new option for treating gram-positive infections. Oncology (Williston Park) 2000;14:23-9. [PubMed]
- Fung HB, Kirschenbaum HL, Ojofeitimi BO. Linezolid: an oxazolidinone antimicrobial agent. Clin Ther 2001;23:356-91. [Crossref] [PubMed]
- Vrioni G, Tsiamis C, Oikonomidis G, et al. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: current achievements and future perspectives. Ann Transl Med 2018;6:240. [Crossref] [PubMed]
- MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother 2003;51 Suppl 2:ii17-25. [Crossref] [PubMed]
- Cho YS, Lim HS, Lee SH, et al. Pharmacokinetics, Pharmacodynamics, and Tolerability of Single-Dose Oral LCB01-0371, a Novel Oxazolidinone with Broad-Spectrum Activity, in Healthy Volunteers. Antimicrob Agents Chemother 2018;62. [Crossref] [PubMed]
- Slatter JG, Stalker DJ, Feenstra KL, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab Dispos 2001;29:1136-45. [PubMed]
- Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet 2003;42:1129-40. [Crossref] [PubMed]
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285-92. [Crossref] [PubMed]
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10-52. [Crossref] [PubMed]
- Cattaneo D, Gervasoni C, Cozzi V, et al. Therapeutic drug management of linezolid: a missed opportunity for clinicians? Int J Antimicrob Agents 2016;48:728-31. [Crossref] [PubMed]
- Pea F, Cojutti PG, Baraldo M. A. 10-Year Experience of Therapeutic Drug Monitoring (TDM) of Linezolid in a Hospital-wide Population of Patients Receiving Conventional Dosing: Is there Enough Evidence for Suggesting TDM in the Majority of Patients? Basic Clin Pharmacol Toxicol 2017;121:303-8. [Crossref] [PubMed]
- Pea F, Furlanut M, Cojutti P, et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother 2010;54:4605-10. [Crossref] [PubMed]
- Zoller M, Maier B, Hornuss C, et al. Variability of linezolid concentrations after standard dosing in critically ill patients: a prospective observational study. Crit Care 2014;18:R148. [Crossref] [PubMed]
- Blassmann U, Roehr AC, Frey OR, et al. Decreased Linezolid Serum Concentrations in Three Critically Ill Patients: Clinical Case Studies of a Potential Drug Interaction between Linezolid and Rifampicin. Pharmacology 2016;98:51-5. [Crossref] [PubMed]
- Muzevich KM, Lee KB. Subtherapeutic linezolid concentrations in a patient with morbid obesity and methicillin-resistant Staphylococcus aureus pneumonia: case report and review of the literature. Ann Pharmacother 2013;47:e25. [Crossref] [PubMed]
- Nukui Y, Hatakeyama S, Okamoto K, et al. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother 2013;68:2128-33. [Crossref] [PubMed]
- Cattaneo D, Alffenaar JW, Neely M. Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin Drug Metab Toxicol 2016;12:533-44. [Crossref] [PubMed]
- Dong HY, Xie J, Chen LH, et al. Therapeutic drug monitoring and receiver operating characteristic curve prediction may reduce the development of linezolid-associated thrombocytopenia in critically ill patients. Eur J Clin Microbiol Infect Dis 2014;33:1029-35. [Crossref] [PubMed]
- Rao GG, Konicki R, Cattaneo D, et al. Therapeutic Drug Monitoring Can Improve Linezolid Dosing Regimens in Current Clinical Practice: A Review of Linezolid Pharmacokinetics and Pharmacodynamics. Ther Drug Monit 2020;42:83-92. [Crossref] [PubMed]
- Lovering AM, Le Floch R, Hovsepian L, et al. Pharmacokinetic evaluation of linezolid in patients with major thermal injuries. J Antimicrob Chemother 2009;63:553-9. [Crossref] [PubMed]
- Sazdanovic P, Jankovic SM, Kostic M, et al. Pharmacokinetics of linezolid in critically ill patients. Expert Opin Drug Metab Toxicol 2016;12:595-600. [Crossref] [PubMed]
- Andes D, van Ogtrop ML, Peng J, et al. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 2002;46:3484-9. [Crossref] [PubMed]
- Rayner CR, Forrest A, Meagher AK, et al. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet 2003;42:1411-23. [Crossref] [PubMed]
- Srinivas NR, Syed M. Applicability of a Single Time Point Strategy for the Prediction of Area Under the Concentration Curve of Linezolid in Patients: Superiority of Ctrough- over Cmax-Derived Linear Regression Models. Drugs R D 2016;16:69-79. [Crossref] [PubMed]
- Pea F, Viale P, Cojutti P, et al. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother 2012;67:2034-42. [Crossref] [PubMed]
- Boak LM, Rayner CR, Grayson ML, et al. Clinical population pharmacokinetics and toxicodynamics of linezolid. Antimicrob Agents Chemother 2014;58:2334-43. [Crossref] [PubMed]
- Bhalodi AA, Papasavas PK, Tishler DS, et al. Pharmacokinetics of intravenous linezolid in moderately to morbidly obese adults. Antimicrob Agents Chemother 2013;57:1144-9. [Crossref] [PubMed]
- Xie F, Mantzarlis K, Malliotakis P, et al. Pharmacokinetic evaluation of linezolid administered intravenously in obese patients with pneumonia. J Antimicrob Chemother 2019;74:667-74. [Crossref] [PubMed]
- Taubert M, Zoller M, Maier B, et al. Predictors of Inadequate Linezolid Concentrations after Standard Dosing in Critically Ill Patients. Antimicrob Agents Chemother 2016;60:5254-61. [Crossref] [PubMed]
- Morata L, Cuesta M, Rojas JF, et al. Risk factors for a low linezolid trough plasma concentration in acute infections. Antimicrob Agents Chemother 2013;57:1913-7. [Crossref] [PubMed]
- Bolhuis MS, van Altena R, Uges DR, et al. Clarithromycin significantly increases linezolid serum concentrations. Antimicrob Agents Chemother 2010;54:5418-9. [Crossref] [PubMed]
- Okazaki F, Tsuji Y, Seto Y, et al. Effects of a rifampicin pre-treatment on linezolid pharmacokinetics. PLoS One 2019;14:e0214037. [Crossref] [PubMed]
- Gervasoni C, Simonetti FR, Resnati C, et al. Prolonged inductive effect of rifampicin on linezolid exposure. Eur J Clin Pharmacol 2015;71:643-4. [Crossref] [PubMed]
- Hashimoto S, Honda K, Fujita K, et al. Effect of coadministration of rifampicin on the pharmacokinetics of linezolid: clinical and animal studies. J Pharm Health Care Sci 2018;4:27. [Crossref] [PubMed]
- Cojutti P, Crapis M, Bassetti M, et al. Linezolid underexposure in a patient co-treated with venlafaxine. Eur J Clin Pharmacol 2015;71:1285-6. [Crossref] [PubMed]
- Pea F, Cadeo B, Cojutti PG, et al. Linezolid underexposure in a hypothyroid patient on levothyroxine replacement therapy: a case report. Ther Drug Monit 2014;36:687-9. [Crossref] [PubMed]






