
IntegrinB5 upregulated by HER2 in gastric cancer: a promising biomarker for liver metastasis
Introduction
Gastric cancer (GC) is one of the most common and lethal cancers worldwide, especially in China (1,2). Like other cancers, metastasis is the leading cause of patients’ death in GC, with liver as one of the mostly chosen organ (3). Patients with GC liver metastasis (GCLM) suffer from a 5-year survival rate around 5% and a median survival less than 10 months underwent traditional chemotherapy (4). Even though progress has been made, the prognosis of GCLM patients is still poor because of delayed diagnosis and ineffective treatments. Thus, novel prognostic biomarkers and treatment recipes for GCLM is in urgent need.
Recently, the roles that exosomes play during the complex cascades of GC distal metastasis has been noted (5). Exosomes are extracellular vesicles produced by cells with diameters range from 30–150 nm (6). Derived from cells, exosomes are rounded by lipid bilayers, and contain biomolecules such as nucleic acids, proteins and lipids, transducing distal signals and exerting various functions (7). Exosomes could remain stable not only in blood, urine and saliva, but also in cell culture medium (8-11). The stability of exosomes makes them ideal models for clinical diagnosis and lab examinations. Moreover, tumor exosome integrins has been found to determine organotropic metastasis (12). Exosomal integrins α6β4 and α6β1 were found to be associated with lung metastasis, while exosomal integrin αvβ5 was linked to liver metastasis (LM) (12). Although the role of exosomal integrin in organotropic metastasis was revealed using breast cancer and pancreatic cancer as models, it’s hypothesized that exosomal integrins may also facilitate LM in GC.
Integrins are a family composed of 24 transmembrane heterodimers formed by 18α integrin and 8β integrin subunits (13). As multidirectional signaling molecules, integrins have been reported to play controversial yet vital roles along the distal metastasis cascades (14). In breast cancer, the cross talk between integrins and HER2 in the development of metastasis has been abundantly reported. Targeting integrin αvβ6 in addition to trastuzumab improved the survival in xenograft models (14-16). In GC, the overexpression of HER2 indicates obstinacy (17,18). As reported, high expression of HER2 in GC is strongly associated with GCLM (19-21). Congruously, in our preceding studies, we found that the risk of LM is over twice higher in HER2 positive advanced GC (AGC) than that in the negative one, and adding HER2 monoclonal antibody trastuzumab to chemotherapy is effective and safe in clinical practice (21,22). However, HER2 status itself was not an independent prognostic factor in GCLM patients. The reason remains an enigma.
Given the above, in this study, the association between HER2 and integrins in GCLM was confirmed. The significance of exosomal integrins in GCLM prognosis was revealed and hereby we propose the combination of HER2 antibody and integrins inhibitors in GCLM treatment.
Methods
Exosome isolation
Exosomes were isolated from the serum of patients using 3D Medicine exosome isolation reagent (#N3525; 3DMed, Shanghai, China). Briefly, serum samples were centrifuged at 12,000 ×g for 10 min at 4 °C after water bath incubation at 37 °C for 5 min. Supernatants were Equilib rated to ambient temperature, sequentially filtered with a 0.45-µm filter and a 0.22-µm filter respectively. One-fourth volume of exosome isolation reagent (3DMed) was added to and mixed with the filtered supernatant in a clean 1.5 mL tube. The mixture was incubated overnight at 4 °C and centrifuged at 4,700 ×g for 30 min at 4 °C to obtain extracellular vesicles precipitate. The isolated exosomes were resuspended in cOmplete Lysis-M EDTA-free (04719964001; Roche, Basle, Switzerland) with a volume equal to that of the serum supernatant.
Exosomes’ characterization
To characterize the obtained exosomes, western blotting and scanning electron microscopy (SEM) detection were performed. For protein extraction, exosomes were homogenized in RIPA lysis buffer supplemented with proteinase inhibitors. Proteins were separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis in 4–20% polyacrylamide gels (Bio-Rad, WA, USA), electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA), and then incubated with primary anti-CD63 (ab68418; Abcam, Cambridge, UK) and anti-CD9 (ab92726; Abcam) at ambient temperature for 2 h. Horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, CA, USA) were incubated with the PVDF membrane at ambient temperature for 1 h after TBST washing for 3 times. Antibody binding was detected using an enhanced chemiluminescence system in accordance with the manufacturer’s protocol (Tanon-5200Multi; Shanghai, China).
Specimens derived from gastric cancer patients
This study was approved by the Research Ethics Committees of Zhongshan hospital, Fudan University, and written informed patient consents were obtained. A total of 132 gastric cancer patients diagnosed with gastric adenocarcinoma and treated with surgery in Shanghai Zhongshan Hospital, Fudan University from 2009 to 2014 were enrolled. All the patients were without distal metastasis and all the tumors of these patients were resectable at the time of surgery. Clinicopathological information, including age, gender, tumor location, tumor size, Lauren classification, differentiation grade and metastasis organs after surgery (follow-up to July 2018), was collected. Distal metastasis sites were confirmed by magnetic resonance imaging (MRI) or computed tomography (CT).
Cell and reagent
Gastric carcinoma cell lines SGC7901, N87, AGS and MNK28 were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). AKT activator was purchased from Selleck (S7863).
Gastric carcinoma cell lines were maintained in RPMI 1640 (HyClone, Logan, UT, USA) supplemented with 10% FBS (complete medium), and 293T cells were maintained in Dulbecco’s Modified Eagles Medium (HyClone). All cells were cultured in a cell incubator (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C and 5% CO2.
Lentivirus production and stable cell line establishment
Core lentiviral plasmids containing shRNAs targeting HER2/integrin β5 or scrambled sequence as negative control were purchased from Shanghai GeneChem Company Ltd. (Shanghai, China). To generate lentivirus particles, the core plasmids were co-transfected into 293T cells with packaging plasmid psPAX2 and envelope plasmid pMD2.G at a ratio of 4:3:1 using HighGene Transfection reagent (Abclonal, Shanghai, China). After 48 h, lentiviral particles were harvested and added to the medium of target cells pre-treated with polybrene (2 µg/mL, Merck Sigma-Aldrich, Burlington, MA, USA). Stable cell lines were selected by puromycin (2 µg/mL, Merck Sigma-Aldrich) and the knockdown efficiency was detected by quantitative real-time PCR and western blot.
Transwell assays and scratch assays
To assess the influence of β5-integrin on the metastasis of gastric carcinoma cells in vitro, Transwell assays and scratch assays were conducted. For Transwell assays, 2×104 cells suspended in RPMI 1640 (without FBS) were seeded in the upper chamber of a porous Transwell insert (8 µm, 353097, Corning) inserted in a well of a 24 well-plate that already contains 500 µL complete medium. After 24 h incubation, the complete medium was substituted with 4% paraformaldehyde for the fixation of cells for 30 min. Then the fixed cells were stained with 0.5% crystal violet at room temperature for 30 min, followed with washing by PBS for three times. Cells did not migrate to the lower chamber were erased with a cotton-tipped swap. Representative images were taken. For scratch assays, 5×105 cells were seeded in a well of a 6 well-plate with complete medium to form a cell layer. The well was then scratched with a 200 µL pipette tip, washed with PBS for twice to remove floating cells and the complete medium was changed for RPMI 1640 supplemented with 2% FBS to allow cells to migrate. Images were taken right after washing and 24 h later.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD, USA) and then reversely transcribed in to cDNA using the high-capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific) according to the manufacturer’s instruction. qRT-PCR was performed using TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, Dalian, China) in a Real-time System (Bio-Rad, Hercules, CA, USA). Samples were run in triplicate and the results were normalized against GAPDH and calibrated to the control.
Western blotting
Cells were lysed using RIPA buffer supplemented with a phosphatase inhibitor cocktail and a protease inhibitor cocktail (Beyotime, Shanghai, China), and cell lysates were quantified using the BCA assay kit (Beyotime, Shanghai, China). Electrophoresis was performed to separate the proteins which were then transferred to PVDF membrane. The membranes were blocked with 5% BSA/TBST and probed with the following primary antibodies: anti-phosphorylated HER2 (#2243, Cell Signaling Technology, Beverly, MA, USA), anti-HER2 (#2165, Cell Signaling Technology), anti-Akt (#4691, Cell Signaling Technology), anti-phosphorylated Akt (#4060, Cell Signaling Technology), anti-ITGβ5 (sc-14010, Santa Cruz), anti-actin (sc-58673, Santa Cruz). After being washed by TBST for 3 times, the membranes were then incubated with secondary antibodies: HRP-conjugated anti-rabbit IgG (#7074, Cell Signaling Technology) or anti-mouse IgG (#7076, Cell Signaling Technology). After the unbound secondary antibodies were washed, the membrane was developed with Super ECL Detection Reagent (Yeason Biotechnology, Shanghai, China), and images were captured using ImageQuant™ LAS 4000 biomolecular imager (GE Healthcare).
Immunohistochemistry (IHC)
IHC was performed on Leica Bond III, as previously described. In brief, tissue samples were paraffin-embedded and sectioned (4.5 µm). After antigen retrieval, sections were incubated with the αV (#CY6887, Abways Technology, Shanghai, China), β5 (#CY1092, Abways Technology) integrin and HER2 (790-4493, Ventana Medical Systmes, AZ, USA) primary antibodies at 4 °C overnight in a humidified chamber. After washing with PBS, the slides were subsequently incubated with the secondary antibodies for 30 minutes, developed using GTvision TM III (Gene Technology, Shanghai, China), and counterstained with hematoxylin. Substitution of the primary antibody with PBS was used as a negative control for tissue section.
ELISA
For the detection of exosomal HER2 in gastric cancer patients’ serum, the total exosomes were isolated by the ExoQuick (SBI, EXOQ5A-1). Briefly, blood cells and debris were removed by centrifugation steps at 3,000 rpm for 10 min and then 13,000 g for 15 min. Next the 250 µL of serum was incubated with 63 µL ExoQuick exosome precipitation solution at 4 °C, for 30 min. Following centrifugation at 1,500 g for 30 min, the exosomes were precipitated and then resuspended in 50 µL cOmplete Lysis-M EDTA-free (Roche, 04719964001). Then the exosomal HER2 expression was assessed by the Human ErbB2/Her2 Quantikine ELISA Kit was used (R&D, DHER20) according to the recommendation of the manufacturer.
Ethics
Patients with advanced gastric cancer who provided blood sample signed written informed consents in our study. This study was approved by the Research Ethics Committees of Zhongshan Hospital, Fudan University.
Statistical analysis
The Student’s t-test was used to compare the gene expression of integrin subtypes in different gastric cancer cell lines and any other comparation of numeric data. χ2 analysis was used to assess the association between integrin and metastasis sites. Survival curves were estimated by the Kaplan-Meier method and a multivariate analysis on survival was using the Cox regression method. OS is defined as the time from a patient diagnosed of gastric cancer according to the pathology to the death. DFS is defined as the time from a patient diagnosed of gastric cancer according to the pathology to the disease recurrence or metastasis. P value <0.05 was considered statistically significant.
Results
Integrin β5 expression is positively correlated with HER2 expression in GC cells
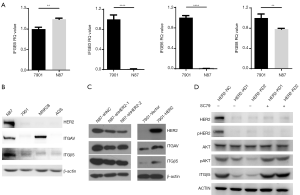
Both integrin αvβ5 and HER2 were reported to be associated with cancer LM. To reveal the association between HER2 and integrin αvβ5 in GC, we detected the expression of integrin αv (ITGAV) associated subunits, integrin β3, β5, β6 and β8 (i.e., ITGβ3, ITGβ5, ITGβ6 and ITGβ8) using qRT-PCR in HER2 positive (N87) and HER2 negative (SGC7901) GC cell lines. As shown in Figure 1A, ITGβ5 is significantly highly transcribed in HER2 positive N87 cells compared with HER2 negative SGC7901 cells (P<0.01). Western blotting was used to further detect the protein levels of ITGβ5 in more GC cells. ITGβ5 showed higher expression in HER2 positive N87 cells compared with HER2 negative SGC7901, MNK28 and AGS cells, regardless of the relative high expression of ITGAV in MNK28 cells (Figure 1B). To verify the association between ITGβ5 and HER2, we knocked down HER2 in N87 cells and overexpressed HER2 in SGC7901 cells. As hypothesized, knocking down of HER2 lead to a notable decrease of ITGβ5 in N87 cells, and overexpression of HER2 brought about an increase of ITGβ5 in SGC7901 cells (Figure 1C). To explore how HER2 regulates ITGβ5, we detected the downstream signals of HER2 and found that knocking down of HER2 diminished AKT phosphorylation (Figure 1D). Besides, AKT activator SC79 increased the expression of ITGβ5 which is reduced by HER2 knockdown, suggesting that the regulation of ITGβ5 by HER2 may due to the regulation of PI3K-AKT pathways (Figure 1D). These results indicate that HER2 upregulates ITGβ5 through PI3K-AKT pathway.
ITGβ5 promotes HER2 positive GC metastasis in vitro
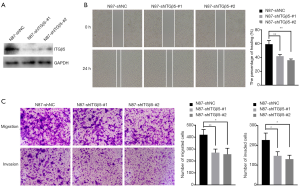
Integrins play an important role in the process of cell adhesion and invasion. According to the above results in vitro, we found that ITGβ5 was associated with HER2 expression in gastric cancer, so we knocked down TIGB5 in HER2 positive N87 cells (Figure 2A) and performed scratch wound healing assay and Transwell assay to assess the role of ITGβ5 in HER2 positive GC metastasis. As shown in Figure 2B, silencing ITGβ5 significantly impeded the wound healing capacity of N87 cells. Accordingly, the results of Transwell assay showed that both in vitro cell migration and invasion were inhibited significantly by ITGβ5 knockdown (Figure 2C). These results indicated the promoting effect of ITGβ5 in GC cells.
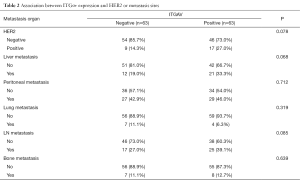
ITGβ5 is associated with LM and prognosis in GC patients
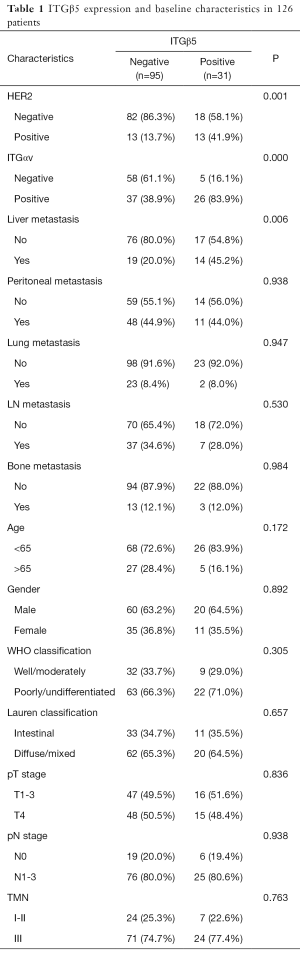
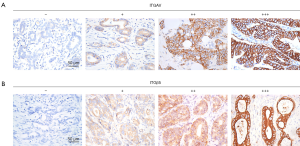
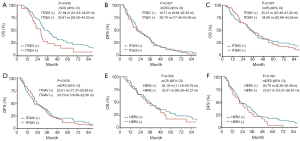
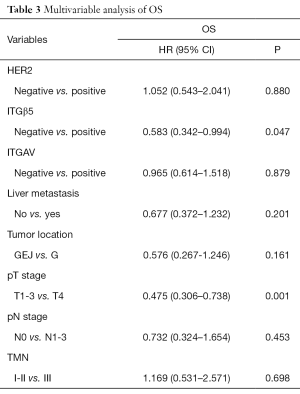
To verify our above analysis of the association between ITGβ5 expression and its clinical significance, we assessed the expression of ITGAvB5 by IHC in 126 cases of gastric cancer who underwent gastrectomy from Feb 2009 to Feb 2015. The patients baseline characteristics were shown in Table 1. ITGAV and ITGβ5 IHC staining patterns in gastric cancer patients are shown in Figure 3A,B respectively. Besides ITGAV. The results of IHC data revealed a strong positive association between the expressions of ITGβ5 and HER2 (P=0.001; Table 1). Until the last follow-up time of June 2018, all patients have disease recurrence or metastasis. A significant association also exists between ITGβ5 expression and GCLM, but not with the other metastatic organs (P=0.006; Table 1). In contrast, significant association was not observed between ITGAV and HER2 or GCLM (Table 2). The 5-year survival rate and 5-year disease free survival (DFS) rate was 23.9% and 11.9% for all 126 patients. Low ITGβ5 expression was not associated with a superior DFS, 5-year DFS rate was 12.9% vs. 10.5% in each group based on ITGβ5 expression (Figure 4). However, overall survival (OS) in patients with high ITGβ5 expression were poorer than patients with low ITGβ5, 5-year survival rate was 12.2% in patients with ITGβ5 high expression while 26.7% for ITGβ5 low expression, while HER2 and ITGAV were not associated with OS (Figure 4). Furthermore, only ITGβ5 and pT stage were independent prognostic factors indicated poor OS in 126 GC patients (Table 3).
Full table
Full table
Full table
The levels of HER2 and ITGβ5 in exosomes are in accordance with that in tissues and behaviors a biomarker for LM
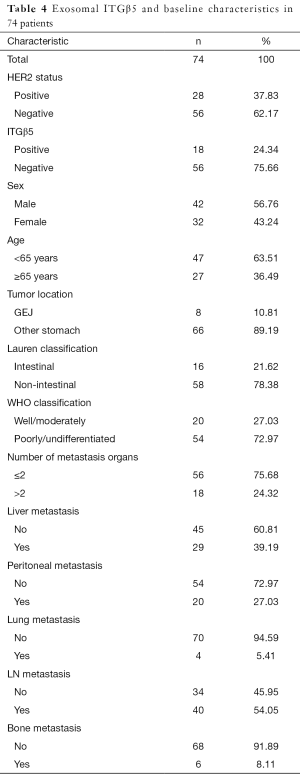
As exosomal protein level often reflect that of the cells, we hypothesized that exosomal HER2 and ITGβ5, which are easily detected in blood, might reveal the levels of HER2 and ITGβ5 in tissues. To test this hypothesis, we first isolated exosomes from the serum of 74 GC patients (Table 4) with distal metastasis (Figure 5A). Then we detected the level of HER2 in tissues and in exosomes by IHC and ELISA respectively. As shown in Figure 5B, the levels of exosomal HER2 in HER2 positive GC patients are dramatically higher than that in HER2 negative patients (P<0.0001). Besides, the association between the levels of exosomal ITGβ5 and that of tissue ITGβ5 showed similar pattern with HER2 (Figure 5C, P<0.001). Above data demonstrated that the levels of exosomal protein might be a substitute with the same clinical significance as what was expressed in tissue. ITGβ5 could reveal the levels of ITGβ5 in tissues, making it feasible to detect the levels of ITGβ5 in GC patients through liquid biopsy.
Full table
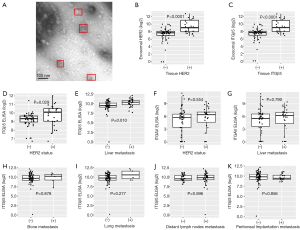
In addition, to explore the possibility of detecting exosomal integrins to predict GCLM, we further assess the association among exosomal ITGAvB5, HER2 status and GCLM. In accordance with the above in IHC results, in the serum of GC patients, the level of exosomal ITGβ5 was significantly higher in HER2 positive GC patients than that in HER2 negative GC patients, which again verified the association between ITGβ5 and HER2. Exosomal ITGβ5 is significantly positively correlated to the status of HER2 (Figure 5D, P=0.0029). Besides, high level of exosomal ITGβ5 in blood is significantly associated with GCLM (Figure 5E, P=0.01). While ITGAV is neither correlated with HER2 status nor GCLM (Figure 5F,G). To verify the specificity of ITGβ5 being correlated with GCLM, we analyzed the association between the levels of exosomal ITGβ5 and other target organs of GC metastasis. The results showed that high level of exosomal ITGβ5 is not correlated with GC bone metastasis, lung metastasis, lymphatic metastasis, or peritoneal implantation metastasis (Figure 5H,I,J,K). Collectively, these data indicate that high ITGβ5 expression is more likely to indicate LM than other organotropic metastases in HER2 positive GC patients.
Discussion
GCLM still remains a challenge in GC treatment despite of the development of GC research in the recent decade (23). We and others congruously found that high expression of HER2 in GC is strongly correlated with GCLM (19-21), yet the benefits from targeting HER2 for GCLM treatment is rather limited. The lack of an ideal model for the prediction and treatment for GCLM results in delayed diagnosis and poor prognosis for GCLM.
In this study, focusing on GCLM, we found the association between HER2 and ITGβ5, a subunit of ITGAvB5. Exosomal ITGAvB5 was reported to be associated with LM in breast cancer and pancreatic cancer (12). In our study, ITGβ5 is highly expressed in HER2 positive GC cell line compared with HER2 negative cell lines. Forced knockdown or overexpression of HER2 changed the levels of ITGβ5 accordingly in GC cells, indicating a positive correlation between HER2 and ITGβ5 expressions, which was found to be possibly linked by AKT pathways. PI3K pathway is one of the main downstream pathways of HER2 and integrin (24,25). The activity of the crosstalk may increase the migration of cell, and promotes resistance in HER2 positive breast cancer (25-27). A consistent result was also confirmed in our study. However, it remains unclear how the expression of ITGβ5 was regulated by activating PI3K pathway with HER2. ITGβ5 plays an essential role in promoting HER2 positive GC cell metastasis in vitro, implying that ITGβ5 would enhance the metastatic potential in HER2 positive GC. However, by contrast to ITGβ5, which only binds to ITGAV, ITGAV was associated with several ITG subtype, including ITGAvB3, ITGAvB5, ITGAvB8 and etc. (28). Our results showed a negative relationship between ITGAV alone and HER2 in vitro.
Although cellular expression of HER2 and ITGβ5 might predict GCLM, it takes complicated steps and long time to acquire IHC results of GC tissues derived from patients. Moreover, liquid biopsy is getting more attention for its advantages as a convenient tool for the diagnosis and prognosis of cancers, including GC (29). The stability of exosome in blood makes it an ideal model for the detection of HER2 and ITGβ5. To assess the feasibility of exosomal HER2 and ITGβ5 as liquid biopsy biomarkers in gastric cancer, we detected the levels of tissue HER2/ITGβ5 and exosomal HER2/ITGβ5 and found a concordance, indicating that the exosomal HER2/ITGβ5 levels would reflect the levels of HER2/ITGβ5 in tissues. Besides, we found that the levels of exosomal ITGβ5 detected by ELISA is positively correlated with that of HER2.
Exosomal ITGAvB5 was reported as a pioneer to liver and created pre-metastasis microenvironment in liver, which indicated exosomal integrin itself played an important role in LM (12). The specificity of ITGβ5 as a liquid biopsy biomarker was also examined in our study. We analyzed the association between exosomal ITGβ5 and GCLM, bone metastasis, lung metastasis, lymphatic metastasis, and peritoneal implantation metastasis. Moreover, another subunit of integrin αvβ5, ITGAV, displays no significant correlation between neither HER2 status or GCLM. The results demonstrated that the level of exosomal ITGβ5 is significantly and exclusively correlated with GCLM, bringing rudiment possibility for ITGβ5 as a specific liquid biopsy biomarker for GCLM. However, determining the cut-off level of ITGβ5 for GCLM prediction awaits a long way to go.
HER2 was reported to be significantly associated with GCLM (19,21). However, previous study found that although adding monoclonal antibody trastuzumab which targets HER2 into therapeutic regimen significantly improved the OS and PFS of HER2 positive advanced GC, the prolonged median OS and PFS were only 3.5 and 1.4 months respectively (22). Thus, to achieve better outcome for HER2 positive GCLM patients, the therapeutic regimen needs to be reasonably reorganized. Our study found ITGβ5 could be a poor predict factor in gastric cancer and had a positive correlation with HER2 and LM. The prognostic value of HER2 was controversial (30,31) and high ITGβ5 expression was reported with poor survival in GC (32). Consistent with previous studies, ITGβ5 was an independent prognostic factor in our study, while HER2 and ITGVA did not show significant correlation with the OS. Integrins are the most predominant cell surface receptors of extracellular matrix proteins, mediating tumor cell adhesion, invasion and proliferation (33,34). Combined with our results in vitro, we hypothesized that ITGβ5 might be associated with the tumor local invasiveness. In previous studies, addition of ITGAvB6 to trastuzumab was effective in HER2 positive breast cancer mouse model and patients (35,36), suggesting combination of trastuzumab and ITGβ5 suppressor/inhibitor for GCLM patients’ therapy may provide a better treatment especially in HER2 positive GCLM. Although integrin inhibitor cilengitide failed to improve glioblastoma patients’ outcome, integrins remain a potential treatment target for cancers (29).
In summary, our study demonstrated that ITGβ5 is expressed and functions in accordance with HER2 in promoting GC metastasis. Exosomal HER2 and ITGβ5 levels reflect the tissue expression levels of HER2 and ITGβ5, and might be a potential specific liquid biopsy biomarker for GCLM. What’s more, we also proposed a combination of HER2 monoclonal antibody trastuzumab and ITGβ5 suppressor/inhibitor for the treatment of GCLM.
Acknowledgments
Clinical sample collecting is supported by standardized training doctors and nurses from Zhongshan Hospital, Fudan University. We gratefully acknowledge Zeng Haiying and Luo Rongkui from the Department of Pathology, Zhongshan Hospital, Fudan University of for immunohistochemical staining and consultation. We thank Liu Qing for assistance with the language checking.
Funding: Research was supported by a Grant from the Science and Technology Commission of Shanghai Municipality (17411951400).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.184). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patients with advanced gastric cancer who provided blood sample signed written informed consents in our study. This study was approved by the Research Ethics Committees of Zhongshan hospital, Fudan University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Riihimaki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016;7:52307-16. [Crossref] [PubMed]
- Picado O, Dygert L, Macedo FI, et al. The Role of Surgical Resection for Stage IV Gastric Cancer With Synchronous Hepatic Metastasis. J Surg Res 2018;232:422-9. [Crossref] [PubMed]
- Fu M, Gu J, Jiang P, et al. Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer 2019;18:41. [Crossref] [PubMed]
- Bardelli A, Pantel K.. Liquid Biopsies, What We Do Not Know (Yet). Cancer Cell 2017;31:172-9. [Crossref] [PubMed]
- Tkach M, Thery C.. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016;164:1226-32. [Crossref] [PubMed]
- Li Q, Shao Y, Zhang X, et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol 2015;36:2007-12. [Crossref] [PubMed]
- Jin Y, Chen K, Wang Z, et al. DNA in serum extracellular vesicles is stable under different storage conditions. BMC Cancer 2016;16:753. [Crossref] [PubMed]
- Street JM, Koritzinsky EH, Glispie DM, et al. Urine Exosomes: An Emerging Trove of Biomarkers. Adv Clin Chem 2017;78:103-22. [Crossref] [PubMed]
- Nair S, Tang KD, Kenny L, et al. Salivary exosomes as potential biomarkers in cancer. Oral Oncol 2018;84:31-40. [Crossref] [PubMed]
- Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329-35. [Crossref] [PubMed]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci 2006;119:3901-3. [Crossref] [PubMed]
- Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 2018;18:533-48. [Crossref] [PubMed]
- Ramovs V, Secades P, Song JY, et al. Absence of integrin alpha3beta1 promotes the progression of HER2-driven breast cancer in vivo. Breast Cancer Res 2019;21:63. [Crossref] [PubMed]
- Eberlein C, Kendrew J, McDaid K, et al. A human monoclonal antibody 264RAD targeting alphavbeta6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene 2013;32:4406-16. [Crossref] [PubMed]
- Jorgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer 2012;3:137-44. [Crossref] [PubMed]
- Cho J, Jeong J, Sung J, et al. A large cohort of consecutive patients confirmed frequent HER2 positivity in gastric carcinomas with advanced stages. Ann Surg Oncol 2013;20 Suppl 3:S477-84. [Crossref] [PubMed]
- Saito T, Nakanishi H, Mochizuki Y, et al. Preferential HER2 expression in liver metastases and EGFR expression in peritoneal metastases in patients with advanced gastric cancer. Gastric Cancer 2015;18:711-9. [Crossref] [PubMed]
- Li Q, Li H, Jiang H, et al. Predictive factors of trastuzumab-based chemotherapy in HER2 positive advanced gastric cancer: a single-center prospective observational study. Clin Transl Oncol 2018;20:695-702. [Crossref] [PubMed]
- Jiang H, Li Q, Yu S, et al. Impact of HER2 expression on outcome in gastric cancer patients with liver metastasis. Clin Transl Oncol 2017;19:197-203. [Crossref] [PubMed]
- Qiu MZ, Li Q, Wang ZQ, et al. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer 2014;134:2468-77. [Crossref] [PubMed]
- Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today 2010;40:287-94. [Crossref] [PubMed]
- Arienti C, Pignatta S, Tesei A.. Epidermal Growth Factor Receptor Family and its Role in Gastric Cancer. Front Oncol 2019;9:1308. [Crossref] [PubMed]
- Sampera A, Sanchez-Martin FJ, Arpi O, et al. HER-Family Ligands Promote Acquired Resistance to Trastuzumab in Gastric Cancer. Mol Cancer Ther 2019;18:2135-45. [Crossref] [PubMed]
- Hanker AB, Estrada MV, Bianchini G, et al. Extracellular Matrix/Integrin Signaling Promotes Resistance to Combined Inhibition of HER2 and PI3K in HER2(+) Breast Cancer. Cancer Res 2017;77:3280-92. [Crossref] [PubMed]
- Furmento VA, Marino J, Blank VC, et al. Granulocyte colony-stimulating factor (G-CSF) upregulates beta1 integrin and increases migration of human trophoblast Swan 71 cells via PI3K and MAPK activation. Exp Cell Res 2016;342:125-34. [Crossref] [PubMed]
- Mathai RA, Vidya RVS, Reddy BS, et al. Potential Utility of Liquid Biopsy as a Diagnostic and Prognostic Tool for the Assessment of Solid Tumors: Implications in the Precision Oncology. J Clin Med 2019. [Crossref] [PubMed]
- Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1100-8. [Crossref] [PubMed]
- Pazo Cid RA, Anton A. Advanced HER2-positive gastric cancer: current and future targeted therapies. Crit Rev Oncol Hematol 2013;85:350-62. [Crossref] [PubMed]
- Wang HB, Liao XF, Zhang J. Clinicopathological factors associated with HER2-positive gastric cancer: A meta-analysis. Medicine (Baltimore) 2017;96:e8437. [Crossref] [PubMed]
- Boger C, Warneke VS, Behrens HM, et al. Integrins alphavbeta3 and alphavbeta5 as prognostic, diagnostic, and therapeutic targets in gastric cancer. Gastric Cancer 2015;18:784-95. [Crossref] [PubMed]
- Ganguly KK, Pal S, Moulik S, et al. Integrins and metastasis. Cell Adh Migr 2013;7:251-61. [Crossref] [PubMed]
- Vogetseder A, Thies S, Ingold B, et al. alphav-Integrin isoform expression in primary human tumors and brain metastases. Int J Cancer 2013;133:2362-71. [Crossref] [PubMed]
- Wu YJ, Muldoon LL, Gahramanov S, et al. Targeting alphaV-integrins decreased metastasis and increased survival in a nude rat breast cancer brain metastasis model. J Neurooncol 2012;110:27-36. [Crossref] [PubMed]
- Moore KM, Thomas GJ, Duffy SW, et al. Therapeutic targeting of integrin alphavbeta6 in breast cancer. J Natl Cancer Inst 2014. [Crossref] [PubMed]


