New peptidendrocins and anticancer chartreusin from an endophytic bacterium of Dendrobium officinale
Introduction
Endophytic microorganisms reside in the living tissues of the host plant and do so in a variety of relationships ranging from symbiotic to pathogenic (1,2). Since the report of taxol and taxane produced by a fungal endophyte from the phloem (inner bark) of Pacific yew in 1993 (3), endophyte has served as a widely used model for the discovery of natural products, with participating in biochemical pathways and produce analogous or novel bioactive compounds (1,4-7). Therefore, the prospects of finding new drugs from endophytes for treating newly developing diseases in humans, plants, and animals are great. Compared with higher organisms, microorganisms can be easily maintained under laboratory conditions, ensuring a constant and inexpensive supply of their secondary metabolites. Moreover, many compounds originally believed to be produced by medicinal plants have been found to be produced by host-associated microorganisms (3,6).
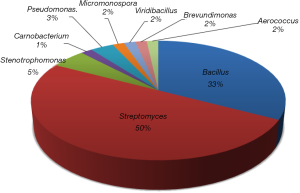
Dendrobium officinale, a tonic herb in Chinese materia medica and health food in folk, has been utilized for the treatment of Yin-deficiency diseases for thousands of years, and was popularly consumed as a functional dietary supplement and one of the most expensive tea materials (8). Modern pharmacological researches have demonstrated that Dendrobium genus have multiple biological activities, including hepatoprotective, anticancer, antimicrobial, hypoglycemic, antifatigue and gastric ulcer protective effects (9-11). As part of an effort to explore the microbial diversity and corresponding metabolic potential of actinomycetes associated with unique ecological environments (12-18), 75 endophytic bacteria (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf) were pure cultured from different parts of D. officinale and submitted for cytotoxic and antibacterial activity screening. After the dereplication by their morphological features and LC-MS screening, 58 total diverse strains were then identified through phylogenetic analysis. The 16S rRNA analysis revealed that the 58 strains represent 9 genera including 4 Actinobacterial genera (Figures 1, http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf), and 50% of them belonged to Streptomyces. According to the bioactivity results (for testing both antibacterial and cytotoxic activities) and LC-HRMS analyses, SH-1.2-R-15 was select as a potentially antibacterial strain for the next scale-up fermentation and secondary metabolites study. Bioassay-guided isolation yielded two new peptides, peptidendrocins A and B [1,2], together with bioactive chartreusin [3], and four other known compounds (Figure 2). Chartreusin [3] showed moderate cytotoxicity against Hep3B2.1-7, J82 and H1299 cancer cell lines, and antibacterial activity against S. aureus. Herein we report the strain isolation, fermentation, isolation, structure elucidation and bioactivity assay of the strains and natural products.
Methods
General experimental procedures
NMR spectra were measured on a Bruker Avance AV500 spectrometer (Bruker, Germany). LC-MS was conducted with an Agilent 1290-6120 LC-Quadrupole MSD mass spectrometer (Agilent Technologies, USA). HRESIMS spectra were obtained on a Q-Exactive Plus mass spectrometer (Thermo Scientific, USA). HPLC analyses were performed on an Agilent 1260 HPLC with PDA detector (Agilent Technologies, USA). Preparative HPLC was performed on a Waters 150 LC system by using a Ultimate®AQ-C18 column (250 × 21.2 mm, 5 µm, Welch Material, USA). MCI gel High porous polymer (75-150 µm) was purchased from Mitsubishi chemical corporation (Japan). Sephadex LH-20 (25-100 µm) was obtained from GE Healthcare (Sweden). XAD16N resin (20-60 mesh) was obtained from Yuanye (Nanjing, Jiangsu, China). TaKaRa Ex Taq DNA Polymerase (USA) and OMEGA gel extraction kit (200) were purchased from Kaimoer (Nanjing, China). TLC silica gel plates (HSGF254) were obtained from Juyou company (Shandong, China). Chemicals were purchased from Acros or Juyou and used without further purification unless otherwise noted.
Strain isolation
Plant samples of D. officinale with two different growth ages were collected from Zhejiang Haixing Biotech Company (Wuyi City, Zhejiang Province, China). The root, leaf and stem of plants were separated and cleaned with water then rinsed in 0.1% Tween-20 for 30 s, sequentially immersed in 75% ethanol for 5 min and in 2% sodium hypochlorite for 5 min and rinsed with 10% NaHCO3 for 10 min to inhibit fungal growth (2). After each treatment, samples were rinsed three times in sterile water. The surface sterilized samples were aseptically dissected into small pieces. 0.5 g of each sample was suspended in 1.0 mL of sterile H2O, and heated at 75 °C for 1 min to eliminate nonsporulating bacteria. A 100 µL aliquot of supernatant was streaked on oatmeal agar and on ISP4 agar plates supplemented with nalidixic acid (25 µg/mL) and amphotericin B (25 µg/mL). A number of sporulating bacterial colonies were observed after 1–2 month of incubation at 28 °C, and each colony was subsequently purified on a M2 agar plate (17). Overall, 58 endophytic strains were isolated from 4 different plant samples as evidenced by morphological features (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf) and 16S rRNA analysis (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf).
Phylogenetic analysis
Each strain was inoculated in 100 mL baffled flask with 20 mL TSB broth. After 3 days culture at 28 °C with 160 rpm agitation, cells were recovered via centrifugation (12,000 rpm for 15 min at 4 °C) and used for genomic DNA isolation using DNA Isolation Kit. The partial 16S rRNA (19) gene fragment was amplified using universal primers (Forward 5'-CAGAGTTTGATCCTGGCT-3'; Reverse 5'-AGGAGGTGATCCAG CCGCA-3') and the desired PCR-amplified product isolated using the OMEGA gel extraction kit (200). 16S rRNA gene sequences were compared with GenBank database using BlastN (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for searching the closest match sequence. The sequences of strain 16S rRNA have been deposited in the NCBI nucleotide database (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf).
Strain prioritization
Individual bacterial colonies were fermented in 50 mL of medium Bran (corn flour, 40 g/L; glucose, 10 g/L; gluten powder, 5 g/L; K2HPO4·3H2O, 0.5 g/L; bran, 10 g/L; CaCO3, 2 g/L; (NH4)2SO4, 1 g/L) using 250 mL baffled Erlenmeyer flasks for six days at 28 °C with 200 rpm agitation. 0.5 g XAD-16 resin was added to the culture 24 h prior to harvesting. Each individual culture was transferred to a 50 mL falcon tube and centrifuged at 5,000 rpm for 15 min. The supernatant was discarded and the mycelia-XAD resin portion was washed twice with 30 mL of distilled water (vortexed, centrifuged and discarded). 15 mL methanol was then added and sonicated for 15 min, followed by centrifuge to generate the crude extract after evaporation of supernatant solvent. Each extract was then subjected to cytotoxic (against Hep3B2.1-7 cancer cell) and antimicrobial (against E. coli and S. aureus) activities screening. 28 strains showed potent inhibition more than the hit cutoff (inhibition =11.7%) in Hep3B2.1-7 cell viability assay (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf). And 21 strains showed inhibition against S. aureus more than the hit cutoff (36.2%) (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf). Only one strain was active against E. coli (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf). The bioactivity combined with LC-MS profiles (used for identification of potential novel secondary metabolites) decided strain SH-1.2-R-15 from the root of one year old D. officinale, to be the most interesting strain capable of producing novel metabolites, based on SciFinder database comparison, and its potent cytotoxic and antimicrobial activity (23.97% and 72.75% inhibition, respectively).
Fermentation, extraction and isolation
Streptomyces sp. SH-1.2-R-15 was cultivated on M2-agar (glucose, 10.0 g/L; malt extract, 10.0 g/L; yeast extract, 4.0 g/L; agar, 15.0 g/L) plates at 28 °C for 7 days. Chucks of agar with the fully-grown strain were used to inoculate five 250 mL Erlenmeyer baffled flasks, each containing 50 mL of medium Bran [corn flour, 40.0 g/L; glucose, 10.0 g/L; gluten powder, 5.0 g/L; K2HPO4·3H2O 0.5 g/L; bran 10.0 g/L; CaCO3 2.0 g/L; (NH4)2SO4 1.0 g/L]. After three days of incubation at 28 °C with 200 rpm agitation, the seed cultures were used to inoculate 80 flasks (250 mL), each containing 100 mL of medium Bran. The fermentation was continued at 28 °C with 200 rpm agitation. After 7 days, the culture broth was combined and centrifuged at 8,000 rpm for 20 min (4 °C). The biomass (mycelium) was extracted with MeOH (3 ´ 1,000 mL) and the corresponding organics were evaporated in vacuo to yield 54.8 g of mycelium crude extract. The supernatant was mixed with 4% (w/v) XAD-16 resin and stirred overnight, followed by filtration. The resin was washed with H2O (3 ´ 500 mL) and then extracted with MeOH until the eluent was colorless. The MeOH extract was subsequently evaporated to afford 27.5g of XAD-crude extract. HPLC and TLC analysis revealed a similar metabolite profile in both mycelial cake and culture extracts. Therefore, the extracts were combined to afford 82.3 g and then fractionated by MCI column (500 g) using gradient elution from 20% to 100% aqueous MeOH to provide nine fractions (A–I). Fraction B (1.51 g) was subjected to Sephadex LH-20 column (80% aqueous MeOH) to obtain two sub-fractions, B1–B2. Sub-fraction B1 (147 mg) was further purified by prep-HPLC (13% aqueous CH3CN) to yield compounds 5 (2 mg, tR =15.2 min) and 6 (3 mg, tR =15.7 min). Fraction D (1.95 g) was subjected to Sephadex LH-20 column (80% aqueous MeOH) to obtain two sub-fractions, D1-D2. Sub-fraction D1 (120 mg) was further purified by prep-HPLC (11% aqueous CH3CN) to yield compound 4 (29 mg, tR =16.0 min). Sub-fraction D2 (206 mg) was further purified by prep-HPLC (30–70% aqueous CH3CN) to yield compounds 1 (1 mg, tR =25.4 min) and 2 (16 mg, tR =25.8 min). Fraction F (1.24 g) was purified by prep-HPLC (50–80% aqueous CH3CN) to yield compound 7 (6 mg, tR =16.7 min). Fraction H (0.99 g) was crystallized in methanol to yield compound 3 (62 mg, tR =25.7 min).
Peptidendrocin A [1]: yellow-brown oily substance. NMR data, see Table 1; (+)-ESI-MS: m/z 409.2 [M + H]+ ; (−)-ESI-MS: m/z 407.2 [M − H]−; (+)-HR-ESI-MS m/z = 409.2444 [M + H]+ (calcd. for C20H33N4O5, 409.2451), (−)-HR-ESI-MS m/z = 407.2294 [M − H]− (calcd. for C20H31N4O5, 407.2294).

Full table
Peptidendrocin B [2]: yellow-brown oily substance. NMR data, see Table 1; (+)-ESI-MS: m/z 423.2 [M + H]+ ; (−)-ESI-MS: m/z 421.2 [M − H]−; (+)-HR-ESI-MS m/z =423.2596 [M+H]+ (calcd. for C21H35N4O5, 423.2607), (−)-HR-ESI-MS m/z =421.2451 [M − H]− (calcd. for C21H33N4O5, 421.2451).
Chartreusin [3] (20,21): pale green powder, HRESIMS m/z 663.1688 [M + Na] + (calcd. for C32H32O14Na+, 663.1690). 1H NMR (500 MHz, DMSO-d6) δ 8.09 (1H, d, J =8.3 Hz, H-8), 7.73 (1H, t, J =8.3 Hz, H-9), 7.53 (1H, d, J =8.3 Hz, H-10), 7.70 (1H, d, J =8.3 Hz, H-16), 7.63 (1H, d, J =8.3 Hz, H-17), 2.82 (3H, s, H-19), 5.38 (1H, d, J =7.7 Hz, H-1’), 3.96 (1H, m, H-2’), 3.63 (1H, m, H-3’), 4.58 (1H, d, J =6.6 Hz, 3’-OH), 3.62 (1H, m, H-4’), 4.89 (1H, d, J =3.8 Hz, 4’-OH), 3.94 (1H, m, H-5’), 0.99 (3H, d, J =6.3 Hz, H-6’), 5.43 (1H, d, J =3.7 Hz, H-7’), 3.37 (1H, m, H-8’), 3.60 (1H, brs, 8’-OH), 3.09 (1H, dd, J =10.1, 2.6 Hz, H-9’), 3.60 (1H, m, H-10’), 4.24 (1H, d, J =4.4 Hz, 10’-OH), 4.21 (1H, m, H-11’), 1.21 (3H, d, J =6.3 Hz, H-12’), 3.13 (3H, s, H-13’); 13C NMR (125 MHz, DMSO-d6) δ 158.8 (C-1), 117.2 (C-2), 120.0 (C-3), 108.7 (C-4), 97.3 (C-5), 155.6 (C-6), 126.3 (C-7), 116.5 (C-8), 128.5 (C-9), 114.5 (C-10), 154.3 (C-11), 118.2 (C-12), 138.6 (C, C-13), 164.0 (C, C-14), 146.5 (C, C-15), 121.0 (C-16), 133.1 (C-17), 138.6 (C-18), 21.9 (C-19), 99.6 (C-1’), 78.0 (C-2’), 72.2 (C-3’), 71.4 (C-4’), 70.4 (C-5’), 16.6 (C-6’), 99.9 (C-7’), 67.0 (C-8’), 79.9 (C-9’), 67.6 (C-10’), 65.9 (C-11’), 16.7 (C-12’), 56.0 (C-13’).
(+)-Streptazolin [4] (22,23): pale yellow oil, HRESIMS m/z 208.0971 [M + H]+ (calcd. for C11H14NO3+, 208.0974). 1H NMR (500 MHz, DMSO-d6) δ 1.91 (3 H, d, J =7.3 Hz, H-1), 2.21-2.50 (2 H, m, H-9), 3.40-3.47 (2 H, m, H-10), 4.31 (1 H, d, J=6.5, H-6), 4.59 (1 H, s, H-5), 4.61 (1 H, d, J=6.6, H-4), 6.06 (1 H, m), 6.10 (1 H, q, J=7.2 Hz); 13C NMR (125 MHz, DMSO-d6) δ 15.0 (C-1), 22.5(C-9), 40.1(C-10), 59.0 (C-6), 73.6 (C-4), 81.9 (C-5), 118.3 (C-8), 122.5 (C-2), 139.5 (C-3), 143.7 (C-7), 159.2 (C-11).
Strepchazolin A [5] (24): pale yellow oil, HRESIMS m/z 206.1177 [M – H2O + H]+ (calcd. for C12H16NO2+, 206.1181). 1H NMR (500 MHz, CD3OD) δ 1.37 (3H, d, J =6.5 Hz, H-1), 4.50 (1H, q, J =6.5 Hz, H-2), 5.84 (1H, brs, H-4), 4.56 (1H, m, H-5), 4.31 (1H, brs, H-6), 6.05 (1H, m, H-8), 2.30 (2H, m, H-9), 3.16 (1H, td, J =12.2, 3.2 Hz, H-10a), 3.82 (1H, dt, J =12.2, 3.2 Hz, H-10b), 2.19 (3H, s, H-12); 13C NMR (125 MHz, CD3OD) δ 22.7 (C-1), 64.9 (C-2), 148.7 (C-3), 130.6 (C-4), 81.3 (C-5), 68.6 (C-6), 140.9 (C-7), 118.0 (C-8), 26.1 (C-9), 46.4 (C-10), 175.7 (C-11), 22.9 (C-12).
Strepchazolin B [6] (24): pale yellow oil, HRESIMS m/z 206.1173 [M – H2O + H]+ (calcd. for C12H16NO2+, 206.1181). 1H NMR (500 MHz, CD3OD) δ 1.37 (3H, d, J = 6.5 Hz, H-1), 4.55 (1H, q, J =6.5 Hz, H-2), 5.89 (1H, brs, H-4), 4.57 (1H, m, H-5), 4.31 (1H, brs, H-6), 5.98 (1H, m, H-8), 2.30 (2H, m, H-9), 3.16 (1H, td, J =12.2, 3.2 Hz, H-10a), 3.84 (1H, dt, J =12.2, 3.2 Hz, H-10b), 2.20 (3H, s, H-12); 13C NMR (125 MHz, CD3OD) δ 23.0 (C-1), 65.0 (C-2), 149.4 (C-3), 129.2 (C-4), 81.2 (C-5), 68.6 (C-6), 141.1 (C-7), 117.4 (C-8), 26.1 (C-9), 46.4 (C-10), 175.6 (C-11), 22.9 (C-12).
(R)-(E,E)-2-(l,3-pentadienyl) piperidine [7 ] (25): pale yellow oil, HRESIMS m/z 152.1432 [M + H]+ (calcd. for C10H18N+, 152.1439). 1H-NMR (DMSO-d6, 500 MHz): δ 6.31(1H, dd, J =15.4, 10.5 Hz, H-8), 6.09(1H, dd, J =15.0, 10.6 Hz, H-9), 5.83(1H, m, H-10), 5.52(1H, dd, J = 15.4, 7.2 Hz, H-7), 3.66 (1H, brs, H-2), 2.89, 3.23 (2H, m, H-6), 1.72, 1.52 (2H, m, H-5), 1.82, 1.50 (2H, m, H-3), 1.49, 1.77 (2H, m, H-4), 1.76 (3H, d, J = 6.5 Hz, H-11); 13C-NMR (DMSO-d6, 125 MHz): δ 134.8 (C-8), 132,5 (C-10), 130.7 (C-9), 126.4 (C-7), 57.1 (C-2), 44.0 (C-6), 29.1 (C-3), 21.9 (C-4), 22.0 (C-5), 18.4 (C-11).
Cell culture and 96-well cell viability assay
Human hepatocellular carcinoma cell line Hep3B2.1-7 (ATCC HB-8064), bladder cancer cell J82, and lung cancer cell H1299 were obtained from Cell Bank of Chinese Academy of Sciences (www.cellbank.org.cn), and cultured according to the manufacturer’s suggested protocol. Cancer cells were cultured in flasks in MEM (Part no. 10-010-CVR, Corning) supplemented with 10% fetal bovine serum (Part no. 10270-106, Thermo Fisher), 1× Anti-Anti (part no. 15240-062, Thermo Fisher) at 37 °C, 5% CO2, and 95% relative humidity. Prior to plating, cells were grown to 80% confluence in MEM complete growth media. After washing once with phosphate-buffered saline (PBS), cells were detached by TrypLE Express (part no. 12604021, Thermo Fisher) and centrifuged at 300g for 5 min. Cells were suspended and counted. Two thousand cells in 80 µL of culture media were seeded in 96-well plates (part no. 3603, Corning). After incubation of the assay plates overnight (about 15 h), cells were treated with test samples i.e., crude extracts or compounds (20 µL, final concentration of 50 or 25 µg/mL depends on the solubility of crude extracts) or vehicle (0.25% DMSO). Cell viability was assessed in triplicate after 72 h of incubation using CellTiterGlo reagent according to manufacturer’s instructions. The Tecan Spark microplate reader (Tecan Group Ltd., Swiss) was used to quantitate luminescence signal.
CellTiter-Glo Luminescent Cell Viability Assay is a robust, sensitive assay, widely-used in evaluation of drug activity on cell viability based on quantification of ATP levels in culture (26,27).
Compound activity was normalized on a per-plate basis using the following equation (26).
Test Well refers to those wells with cells treated with test compounds. High Control is defined as wells containing medium only (100% inhibition), and Low Control wells contain cells treated with DMSO only (0% inhibition).
IC50 values of top hits were determined by fitting the concentration–response curve (CRC) data with a four-parameter variable-slope method in GraphPad Prism (GraphPad Software, USA).
Bacterial culture and 96-well turbidity assay
Standard strain of Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were obtained from CICC (China Center of Industrial Culture Collection, China). Bacterial pharmacology of reference controls was verified based on the antibiotic minimum inhibitory concentrations (MICs) determined by the standard broth dilution method according to M7-A7 Clinical and Laboratory Standards Institute (CLSI) guideline. The MIC was defined as the lowest drug concentration that prevented visible bacterial growth following overnight incubation. The MIC determined here using the described absorbance readout method (28) matched the determined MIC according to the CLSI guideline. Bacteria were inoculated in LB Broth media and incubated overnight at 37 °C. Overnight bacterial cultures were quantified via spectrophotometer, diluted to 106 CFU/mL (OD600) and dispensed to 96-well black, clear-bottom assay plates (120 µL/well). This was followed by addition of test samples i.e., crude extracts or compounds (final concentration 200 or 100 µg/mL depends on the solubility of crude extracts) and controls (high control of 100 µg/mL Ampicillin and low control of DMSO only) prepared in 30 µL media (final 0.75% DMSO). The plates were incubated for 15 h at 37 °C. After incubation, absorbance at 600 nm was measured using a Tecan Spark microplate reader. Compound activity was calculated on a per-plate basis similar to the cell viability test.
Results
Four different D. officinale plants with different growth ages (one and three years old), collected from Zhejiang Haixing Biotech (Wuyi City, Zhejiang Province, China), were selected for endophyte culture. After surface sterilization (2), a total of 75 endophytic strains were isolated from the secondary generation plates, and then fermented in bran medium. The cytotoxic (against Hep3B2.1-7 cancer cell) and antimicrobial (against E. coli and S. aureus) activities of 75 crude extracts were then tested. 28 strains of them showed potent inhibition more than the hit cutoff (inhibition =11.7%) in Hep3B2.1-7 cell viability assay (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf). And 21 strains showed inhibition against S. aureus more than the hit cutoff (36.2%) (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf). Only one strain was active against E. coli (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf). According to the bioactivity and LC-MS profiles (used for identification of potential novel secondary metabolites), strain SH-1.2-R-15 was selected for further scale-up fermentation and active metabolites analysis. The crude extract of strain SH-1.2-R-15, which was isolated from the root of one-year old D. officinale, presented both a cytotoxic and antibacterial activity (23.97% and 72.75% inhibition, respectively).
Scale-up fermentation (8 L) of Streptomyces sp. SH-1.2-R-15, followed by extraction, fractionation, and chromatographic purification (http://fp.amegroups.cn/cms/cd8427b7620a53c4c22dc506052aaccb/atm.2020.03.227-1.pdf) yielded two new peptide-type compounds, peptidendrocins A (1, yield: 0.13 mg/L) and B (2, yield: 2.00 mg/L), together with five known compounds: chartreusin (20,21) (3, yield: 7.75 mg/L), (+)-streptazolin (22,23) (4, yield: 3.62 mg/L), strepchazolin A (24) (5, yield: 0.25 mg/L), strepchazolin B (24) (6, yield: 0.38 mg/L), (R)-(E, E)-2-(l,3-pentadienyl)-piperidine (25) (7, yield: 0.75 mg/L).
Structure elucidation
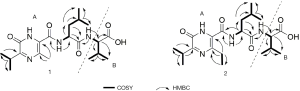
The molecular formula of compound 1 was established as C20H32N4O5 based on the HRESIMS data ([M + H]+, m/z 409.2444). The peptide structure of 1 was evident from the 1H and 13C NMR data recorded in CDCl3 (Table 1), such as the typical NMR chemical shifts for amide and α-methine resonances (δC 176.5 and 172.5, δC/H 57.4/4.50 and 51.5/4.72, respectively). The COSY coupling system of 2'-NH (δH 7.99)/H-2' (δH 4.72)/H2-3' (δH 1.64)/H-4' (δH 1.69)/H3-5' (δH 0.97)/H3-6' (δH 0.95), together with two HMBC correlations of both H-2' and H-3' to a carbonyl (C-1', δC 172.5) indicated the presence of a leucine residue. Similarly, a valine unit was assigned on the basis of interpretation of 2D NMR data. Long-range cross peak from amide proton 2''-NH (δH 7.22) to the carbonyl C-1' established the Leu-Val unit as shown in Figures 2,3. The remaining isopropyl apparent from COSY spectrum, an additional methyl (δH 2.64) and the key HMBC correlations from 2'-NH to two carbons (δC 165.0 and 122.9) established the pyrazinone moiety of 1, thus confirmed the fragment A (Figure 3) as identical with JBIR-57 reported (29). The relative configuration of 1 was also determined by NOESY experiment and comparing the NMR data with literatures. In a similar manner, HRESI-MS and 1D/2D NMR (Table 1) revealed 2 as a C-6 ethyl substituted analogue of 1 with only difference of one CH2 (Figure 2). On basis of this cumulative analysis, 1 and 2 were identified as new pyrazinone peptides and thereby designated peptidendrocins A and B to reflect the producing strain’s point of origin.
Five previously reported compounds, chartreusin [3] (20,21), (+)-streptazolin [4] (22-23), strepchazolins A [5] and B [6] (24), (R)-(E,E)-2-(l,3-penta- dienyl)-piperidine [7] (25) were also identified as fermentation products of Streptomyces sp. SH-1.2-R-15 via comparison to previously reported MS and NMR spectroscopic data.
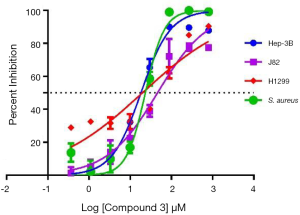
Inspired by the putative antibacterial and anticancer activities reflected from the crude extract, we tested all isolated compounds 1–7 in a similar cytotoxicity (against human hepatoma cancer cell Hep3B2.1-7, bladder cancer cell J82 and lung cancer cell H1299) and antimicrobial assay (against E. coli and S. aureus). While compound 3 displayed potent cytotoxicity (IC50 = 18.19 µM against Hep3B2.1-7, IC50 = 19.74 µM against H1299 and IC50 =45.86 µM against J82) and antibacterial activity against S. aureus with IC50 of 23.25 µM (Figure 4), the new isolates 1–2 and other known compounds were inactive (IC50s >100 µM) in the parallel assays.
The results of activity evaluation for isolated compounds suggested that the primary product, chartreusin, is responsible for the antibacterial and anticancer activities of Streptomyces sp. SH-1.2-R-15. Thus, it will be worthy to prepare more active chartreusin for structure modification to improve the activity, or even for animal study.
Conclusions
In summary, as an effort to explore the microbial diversity and corresponding metabolic potential of endophyte, 58 endophytic bacterial strains, which included 29 Streptomyces sp., were obtained from a dietetic medical plant D. officinale. Fifty-eight strains represented 9 diverse genera, including 4 Actinobacterial genera. The scale-up fermentation followed by bioassay-guided isolation of selected Streptomyces sp. SH-1.2-R-15, led to the discovery of two new peptidendrocins A and B [1 and 2], and chartreusin [3] with moderate cytotoxicity against Hep3B2.1-7 (IC50 =18.19 µM) and H1299 (IC50 =19.74 µM) cancer cell lines, and antibacterial activity against S. aureus (IC50 =23.25 µM), as well as four known secondary metabolites. Endophytes have now become a potential source for the discovery of novel natural products, this study highlights the endophytic bacteria from D. officinale with potential antimicrobial/cytotoxic activity and natural product diversity, and implicates them as a valuable source for new antibiotics and anticancer agents.
Acknowledgments
Funding: This work was financially supported by National Key R&D Program (No. 2019YFC1711500, 2018YFC1707900), Nature Science Foundation of Jiangsu Higher Education Institutions of China (No. 19KJA180008), Jiangsu Provincial “Double Creation Program” (2018), and National Natural Science Foundation of China (Grant No. 81803417, 81903108, 21606062)
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.227). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All activity screening protocols were approved by Hangzhou Normal University, Hangzhou, China.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Strobel G, Daisy B, Castillo U, et al. Natural products from endophytic microorganisms. J Nat Prod 2004;67:257-68. [Crossref] [PubMed]
- Zhao K, Penttinen P, Guan T, et al. The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr Microbiol 2011;62:182-90. [Crossref] [PubMed]
- Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993;260:214-16. [Crossref] [PubMed]
- Gómez OC, Luiz JHH. Endophytic fungi isolated from medicinal plants: future prospects of bioactive natural products from Tabebuia/Handroanthus endophytes. Appl Microbiol Biotechnol 2018;102:9105-19. [Crossref] [PubMed]
- Newman DJ, Cragg GM. Endophytic and epiphytic microbes as "sources" of bioactive agents. Front Chem 2015;3:34. [Crossref] [PubMed]
- Cui Y, Yi D, Bai X, et al. Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 2012;83:913-20. [Crossref] [PubMed]
- Kroiss J, Kaltenpoth M, Schneider B, et al. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol 2010;6:261-3. [Crossref] [PubMed]
- Tang H, Zhao T, Sheng Y, et al. Dendrobium officinale Kimura et Migo: a review on its ethnopharmacology, phytochemistry, pharmacology, and industrialization. Evid Based Complement Alternat Med 2017;2017:7436259.
- Li L, Yao H, Li X, et al. Destiny of Dendrobium officinale Polysaccharide after oral administration: indigestible and nonabsorbing, ends in modulating Gut microbiota. J Agric Food Chem 2019;67:5968-77. [Crossref] [PubMed]
- Liang J, Li H, Chen J, et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol Res 2019;148:104417. [Crossref] [PubMed]
- Devi PU, Selvi S, Devipriya D, et al. Antitumor and antimicrobial activities and inhibition of in-vitro lipid peroxidation by Dendrobium nobile. Afr J Biotechnol 2009;8:2289-93.
- Wang X, Abbas M, Zhang Y, et al. Baraphenazines A-G, divergent fused phenazine-based metabolites from a Himalayan Streptomyces. J Nat Prod 2019;82:1686-93. [Crossref] [PubMed]
- Wang X, Elshahawi SI, Cai W, et al. Bi- and tetracyclic spirotetronates from the coal mine fire isolate Streptomyces sp. LC-6-2. J Nat Prod 2017;80:1141-9. [Crossref] [PubMed]
- Wang X, Elshahawi SI, Shaaban KA, et al. Ruthmycin, a new tetracyclic polyketide from Streptomyces sp. RM-4-15. Org Lett 2014;16:456-9. [Crossref] [PubMed]
- Wang X, Reynolds AR, Elshahawi SI, et al. Terfestatins B and C, new p-terphenyl glycosides produced by Streptomyces sp. RM-5-8. Org Lett 2015;17:2796-9. [Crossref] [PubMed]
- Zhao H, Yang A, Zhang N, et al. Insecticidal endostemonines A−J produced by endophytic Streptomyces from Stemona sessilifolia. J Agric Food Chem 2020;68:1588-95. [Crossref] [PubMed]
- Wang X, Shaaban KA, Elshahawi SI, et al. Frenolicins C-G, pyranonaphtho-quinones from Streptomyces sp. RM-4-15. J Nat Prod 2013;76:1441-7. [Crossref] [PubMed]
- Wang X, Zhang Y, Ponomareva LV, et al. Mccrearamycins A-D, Geldanamycin-derived cyclopentenone macrolactams from an eastern Kentucky abandoned coal mine microbe. Angew Chem Int Ed Engl 2017;56:2994-8. [Crossref] [PubMed]
- Clarridge JE 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 2004;17:840-62. [Crossref] [PubMed]
- Beisler JA. Chartreusin, a glycosidic antitumour antibiotic from Streptomyces. Prog Med Chem 1982;19:247-68. [Crossref] [PubMed]
- Wang YS, Zhang B, Zhu J, et al. Molecular basis for the final oxidative rearrangement steps in chartreusin biosynthesis. J Am Chem Soc 2018;140:10909-14. [Crossref] [PubMed]
- Nomura I, Mukai C. Studies on the total synthesis of streptazolin and its related natural products: first total synthesis of (+/-)-8alpha-hydroxystreptazolone. J Org Chem 2004;69:1803-12. [Crossref] [PubMed]
- Trost BM, Chung CK, Pinkerton AB. Stereocontrolled total synthesis of (+)-streptazolin by a palladium-catalyzed reductive diyne cyclization. Angew Chem Int Ed Engl 2004;43:4327-9. [Crossref] [PubMed]
- Yang CL, Wang YS, Liu CL, et al. Strepchazolins A and B: two new alkaloids from a marine Streptomyces chartreusis NA02069. Mar Drugs 2017;15:244. [Crossref] [PubMed]
- Yang XQ, Yang YB, Zhou H, et al. New megastigmane glycoside and alkaloids from Streptomyces sp. YIM 63342. Nat Prod Res 2013;27:1191-6. [Crossref] [PubMed]
- Kota S, Hou S, Guerrant W, et al. A novel three-dimensional high-throughput screening approach identifies inducers of a mutant KRAS selective lethal phenotype. Oncogene 2018;37:4372-84. [Crossref] [PubMed]
- Wang-Bishop L, Chen Z, Gomaa A, et al. Inhibition of AURKA Reduces Proliferation and Survival of Gastrointestinal Cancer Cells With Activated KRAS by Preventing Activation of RPS6KB1. Gastroenterology 2019;156:662-75.e7. [Crossref] [PubMed]
- Collia D, Bannister TD, Tan H, et al. A rapid phenotypic whole-cell screening approach for the identification of small-molecule inhibitors that counter beta-lactamase resistance in Pseudomonas aeruginosa. SLAS Discov 2018;23:55-64. [Crossref] [PubMed]
- Motohashi K, Inaba K, Fuse S, et al. JBIR-56 and JBIR-57, 2(1H)-pyrazinones from a marine sponge-derived Streptomyces sp. SpD081030SC-03. J Nat Prod 2011;74:1630-5. [Crossref] [PubMed]