
Airway invasive aspergillosis with organizing pneumonia: a case report and review of literature
Introduction
Aspergilla infections account for a large number of mycoses. Among the various clinical presentations of invasive pulmonary aspergillosis, it may also lead to a severe prognosis. The disease manifestation depends on the immune status of the host and the structural integrity of the airways and the lung parenchyma (1).
Organizing pneumonia (OP) is characterized histopathologically by granulation tissue within small airways and alveolar ducts and chronic inflammation in the surrounding alveoli (2). The presenting symptoms are usually nonspecific and include constitutional symptoms with nonspecific radiographic findings. Organizing pneumonia can occur spontaneously and without a known cause; this phenomenon is known as cryptogenic organizing pneumonia (COP) or when associated with diseases known to induce this pathologic pattern, secondary cryptogenic organizing pneumonia (SOP) (3).
Interestingly, SOP may be associated with chronic aspiration, even in patients previously unsuspected to have aspirated (4). Infectious fungi, such as Cryptococcus neoformans (5,6) and Pneumocystis jirovecii (7), may also cause SOP. However, little is known about the relationship between SOP and IPA, and the mechanisms of this linkage have not been elucidated. We present the following case following the Case REpor (CARE) guidelines (8).
Case presentation
In January 2018, A previously healthy 62-year-old man presented to the hospital with a month of intermittent fever accompanied by cough and expectoration. He had caught a common cold after tending to an ancestral tomb one month prior. He denied any medical history of hypertension, coronary heart disease, diabetes, hepatitis, or tuberculosis. The patient was a 30-year 2-pack-a-day smoker but had given up smoking for eight years. He drank 100 grams of liqueur each day. He had no history of industrial or asbestos exposure. Besides this, his other personal and family history was unremarkable.
In December 2017, before admission, he had received antibiotics (for an unspecified condition) for four days in a country hospital with little improvement. He then went to the city hospital for further therapy. The results of the hemogram showed a white blood cell (WBC) count of 16.72×109/L, a neutrophil count of 15.23×109/L (91.1%), and a C-reactive protein (CRP) level of 197 mg/L. A chest computed tomography (CT) scan revealed infection in both lungs. He was treated with cefepime, levofloxacin, and oseltamivir for five days with no effect. His fever was reduced when replacing therapy with biapenem, levofloxacin, and dexamethasone (5 mg). However, his temperature rose after cessation of glucocorticoid for three days. In the city hospital, the sputum culture was positive for Aspergillus. Therefore, the patient was treated with voriconazole and dexamethasone irregularly for three weeks. A later CT scan showed cluster, and patchy shadows remained after antifungal therapy.
On the first admission, his temperature was 37.8 °C, blood pressure was 130/85 mmHg, pulse rate was 78 beats/min, and respiratory rate was 19 breaths/min. Superficial lymph nodes were not enlarged. Breath sounds in 2 lungs were coarse with scattered rhonchi, which was evident during the inhalation end and exhalation phase.
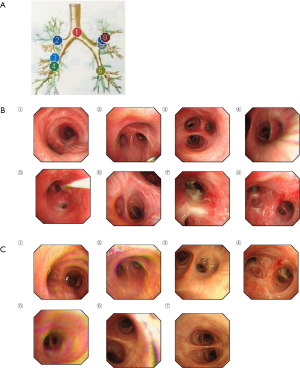
On examination, his WBC count was elevated (12.24×109/L) with a neutrophil count of 10.26×109 ⁄ L (83.9%) and an elevated CRP level (56 mg/L). CT scan revealed cluster and patchy shadows in both lungs, especially the upper lobe of the left lung (Figure 1). G ((1,3)-β-D-polyglucosan) test, galactomannan (GM) test, and enzyme-linked immunospot (ELISPOT) assay for tuberculosis interferon-γ (IFN-γ) were negative. Many sputum smears and cultures of bacteria, fungi, and tuberculosis were all negative. Transbronchial lung biopsy (Figure 2) showed massive necrotic tissues and exudates firmly adhering to the bronchiolar walls. The flushed tissues from bronchiolar walls contained fungal hyphae and spores. Therefore, bronchoalveolar lavage fluid (BALF) was harvested for bacterial culture, which showed he was positive for Staphylococcus aureus. As a result, he was clinically diagnosed as invasive pulmonary aspergillosis and staphylococcus aureus pneumonia. Based on the initial diagnosis of invasive pulmonary aspergillosis as shown by transbronchial lung biopsy, voriconazole (250 mg every 12 h), imipenem and cilastatin sodium (1 g every eight hours), and caspofungin (50 mg every 24 h) was initiated.
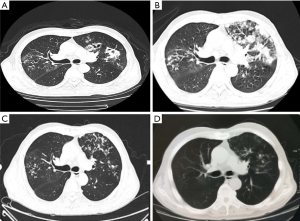
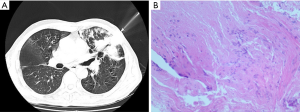
After 15 days of therapy, he still developed a fever. CT re-examination showed an aggravation of cluster and patchy shadows (Figure 1B). His WBC count was still elevated (10.38×109/L) with a neutrophil count of 7.4×109 ⁄ L (71.2%) and an even higher C-reactive protein level (80 mg/L). CT-guided percutaneous lung biopsy was performed in the left upper lung (Figure 3A), and a biopsy specimen revealed interstitial infiltration of neutrophils, lymphocytes, and plasma cells. Local vestal fibrosis, foam cell reaction in the alveolar cavity, and a small number of suspicious fungal substances in the focal area were also recorded in the hematoxylin and eosin (H&E) staining (Figure 3B). Aspergillosis with organizing pneumonia was suspected, taking into consideration the radiological and the histopathological findings, airway invasive. Therefore, the patient received a combinational therapy of voriconazole (200 mg, every 12 h) and methylprednisolone (20 mg, every 24 h).
One week after the initiation of this treatment regimen, the patient’s temperature returned to normal. CT scan suggested that the patchy shadows and large confluent consolidations in both lungs had been alleviated (Figure 3C). Transbronchial lung biopsy confirmed that the bronchiolar walls recovered without neoplasm, congestion, ulcers, or edema (Figure 2C).
Two months after combination therapy, a CT scan indicated the pulmonary consolidations were almost entirely resolved (Figure 1D), and the patient was subsequently discharged. To date, the patient has no sign of fungal infection or pulmonary inflammation.
Discussion and conclusions
This article reports a 62-year-old man diagnosed with invasive pulmonary aspergillosis (IPA) with organizing pneumonia. Based on transbronchial lung biopsy showing fungal hyphae and spores in massive necrotic tissues and BALF showing positive for Staphylococcus aureus, the patient was initially diagnosed with invasive pulmonary aspergillosis and Staphylococcus aureus pneumonia infection. After antibiotics treatment (voriconazole, imipenem, cilastatin sodium, and caspofungin) for 15 days, the patient received CT re-examination. However, the CT scan unexpectedly revealed progressive clustering and patchy shadows. CT-guided percutaneous lung biopsy showed interstitial infiltration of inflammatory cells. Local vestal fibrosis, foam cell reaction in the alveolar cavity, and suspicious fungal substances in the focal area were also recorded in the H&E staining. As a result, invasive airway aspergillosis with organizing pneumonia was suspected. He received the combinational therapy of voriconazole and methylprednisolone. After two months of combination therapy, the CT scan confirmed that the pulmonary consolidations were almost entirely resolved. Therefore, the patient was diagnosed with invasive airway aspergillosis with organizing pneumonia.
Aspergillus causes a broad spectrum of illnesses in humans, which are broadly classified as saprophytic, allergic, or invasive (9) depending on the nature of the relationship between the immune response of the host and the virulence of Aspergillus (1). Classic risk factors for this infection include immunosuppressive therapy, neutropenia, and transplantation. Furthermore, IPA may follow influenza virus infection, emerging as a serious infection in patients with influenza (10). Critically ill patients (11) and those with chronic obstructive pulmonary disease (COPD) (12) and liver disease (13) were found to be susceptible to pulmonary aspergillosis. Cases of IPA have been reported in normal hosts after extensive environmental exposure to aspergillus spores, in the form of moldy hay, tree-bark chippings, near drowning, or inhalation of dust in a mushroom factory. This clinical presentation may be confused with extrinsic allergic alveolitis (EAA), leading to inappropriate treatment with steroids and resulting in clinical deterioration (14). In this case, the previously healthy elderly man visited an ancestral grave and caught a common cold. Initially, the patient was misdiagnosed as EAA and experimentally treated with antibiotics and steroids. Considering that the man was a heavy smoker and a drinker, we could not preclude his respiratory systems being compromised and therefore vulnerable to aspergillosis invasion.
Organizing pneumonia associated with invasive pulmonary aspergillosis has been sporadically reported. In 2014, a patient died from the concomitant occurrence of invasive pulmonary aspergillosis with organizing pneumonia (15). In 2017, a man suffered from chronic pulmonary aspergillosis that led to the development of organizing pneumonia, which was successfully treated with corticosteroid and surgical intervention (16). In 2018, a patient receiving mTOR inhibitor therapy developed invasive pulmonary aspergillosis mimicking organizing pneumonia (17). Together with our cases, these clinical observations indicate that pulmonary aspergillosis may be involved with the pathogenesis of organizing pneumonia.
The co-existence of organizing pneumonia and IPA makes both diagnosis and treatment difficult and challenging. Firstly, cases of IPA in normal hosts are seldom considered, as is commonly the case in immunocompromised patients, such as allogeneic hematopoietic stem cell transplantation recipients, patients presenting with cancer and hematological diseases or treated by anticancer chemotherapy, or solid organ transplantation recipients (18). Secondly, it is also worth considering whether drug reactions could be risk factors for organizing pneumonia (19). Clinicians should maintain a high index of suspicion for invasive pulmonary aspergillosis with organizing pneumonia to avoid delayed diagnosis and therapeutic pitfalls, as this condition is not responsive to antibiotic therapy and should be treated with corticosteroid.
The co-existence of organizing pneumonia with invasive pulmonary aspergillosis is rare. Delayed diagnosis and treatment may have fatal consequences. Transbronchial lung biopsy is of great value in the disease diagnosis. The combinational therapy of voriconazole and methylprednisolone is recommended. Though we successfully treated the patient suffering from SOP with IPA, we did not further investigate the pathogenesis of SOP with IPA. Next-generation sequencing (NGS) may be a suitable method for exploring the risk genes for SOP in IPA patients.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China grant 81770031 and 81671563. The funders had no roles in the design of the study; the collection, analysis, and interpretation of data; or in writing the manuscript.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform (available at http://dx.doi.org/10.21037/atm.2020.03.162) The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Patterson KC, Strek ME. Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest 2014;146:1358-68. [Crossref] [PubMed]
- Cordier JF. Organising pneumonia. Thorax 2000;55:318-28. [Crossref] [PubMed]
- Drakopanagiotakis F, Polychronopoulos V, Judson MA. Organizing pneumonia. Am J Med Sci 2008;335:34-9. [Crossref] [PubMed]
- Mukhopadhyay S, Katzenstein AL. Pulmonary disease due to aspiration of food and other particulate matter: a clinicopathologic study of 59 cases diagnosed on biopsy or resection specimens. Am J Surg Pathol 2007;31:752-9. [Crossref] [PubMed]
- Kessler AT, Al Kharrat T, Kourtis AP. Cryptococcus neoformans as a cause of bronchiolitis obliterans organizing pneumonia. J Infect Chemother 2010;16:206-9. [Crossref] [PubMed]
- Chikumoto A, Oishi K, Hamada K, et al. Cryptococcosis as a cause of organizing pneumonia. Respir Med Case Rep 2019;27:100851. [Crossref] [PubMed]
- Fernandez-Codina A, Caralt-Ramisa B, Masclans JR, et al. An unusual case of organizing pneumonia and infection by P. jirovecii. Med Intensiva 2013;37:299-300. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002;121:1988-99. [Crossref] [PubMed]
- Alshabani K, Haq A, Miyakawa R, et al. Invasive pulmonary aspergillosis in patients with influenza infection: report of two cases and systematic review of the literature. Expert Rev Respir Med 2015;9:89-96. [Crossref] [PubMed]
- Boch T, Reinwald M, Spiess B, et al. Detection of invasive pulmonary aspergillosis in critically ill patients by combined use of conventional culture, galactomannan, 1-3-beta-D-glucan and Aspergillus specific nested polymerase chain reaction in a prospective pilot study. J Crit Care 2018;47:198-203. [Crossref] [PubMed]
- Mohedano Del Pozo RB, Rubio Alonso M, Cuetara Garcia MS. Diagnosis of invasive fungal disease in hospitalized patients with chronic obstructive pulmonary disease. Rev Iberoam Micol 2018;35:117-22. [Crossref] [PubMed]
- Prattes J, Hoenigl M, Krause R, et al. Invasive aspergillosis in patients with underlying liver cirrhosis: a prospective cohort study. Med Mycol 2017;55:803-12. [Crossref] [PubMed]
- Kosmidis C, Denning DW. Republished: The clinical spectrum of pulmonary aspergillosis. Postgrad Med J 2015;91:403-10. [Crossref] [PubMed]
- Xie S, Shen C, Zhang Y, et al. Cryptogenic organizing pneumonia associated with invasive pulmonary aspergillosis: a case report and review of the literature. Int J Clin Exp Pathol 2014;7:8637-46. [PubMed]
- Sakurai A, Yanai H, Ishida T, et al. Possible relationship between organizing pneumonia and chronic pulmonary aspergillosis: A case report and literature review. Respir Investig 2017;55:74-8. [Crossref] [PubMed]
- Iijima Y, Fujioka N, Uchida Y, et al. Invasive pulmonary aspergillosis mimicking organizing pneumonia after mTOR inhibitor therapy: A case report. Int J Infect Dis 2018;69:75-7. [Crossref] [PubMed]
- Lortholary O, Gangneux JP, Sitbon K, et al. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005-2007). Clin Microbiol Infect 2011;17:1882-9. [Crossref] [PubMed]
- Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, et al. Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest 2011;139:893-900. [Crossref] [PubMed]


