
Preoperative leukocytosis is associated with increased tumor-infiltrating neutrophil extracellular traps and worse outcomes in esophageal cancer
Introduction
Esophageal cancer (EC) is the eighth most common cancer and the sixth leading cause of cancer deaths worldwide (1,2). Esophagectomy is a major component of multimodality therapy for resectable EC, and despite the considerable advances in diagnosis and treatment, the 5-year overall survival (OS) for EC remains poor (15% to 35%) (3). Moreover, most patients with EC are resistant to chemotherapy after surgery and relapse within 2 years (4). Thus, a predictive model for the effectiveness and prognosis of chemotherapy in patients with EC is urgently needed.
Clinically, the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system is the most widely used prognostic indicator (5); the accurate staging of EC is integral for determining a suitable treatment plan, but patients who were at the same TNM stage and received similar treatment usually had variable outcomes (6,7). Recently, cancer-related inflammation was identified as a crucial host-related factor that may affect the OS of patients with cancer (8,9). As a predictor of prognosis, serum markers such as lymphocyte counts, neutrophil counts and other indicators are easy to obtain from routine preoperative examinations (10,11). The relationship between leukocytosis and poor prognosis has been confirmed in oropharyngeal cancer and cervical cancer (12,13). However, few studies have considered the association of the peripheral white blood cell (WBC) count and the prognosis of EC, and the optimal cut-off value for WBC has not been determined. In addition, potential mechanism for this phenomenon has not been provided thus far.
As an inflammatory-associated tumor, EC is also characterized by the infiltration of heterogeneous immune cells and peripheral hematologic profile disorders (14). Neutrophils, which serve as the first line of innate immune defense against pathogens, are the main components of WBC, accounting for 50–75% of leukocytes in peripheral blood (15). In 2004, a novel mechanism of neutrophils counteracting the pathogens by forming a DNA based web like structures called neutrophil extracellular traps (NETs) was described (16). The original role of NETs was to trap and kill bacteria, but recent studies have shown that neutrophil activation disorder leads to overproduction of NETs and is positively related to the progression of many diseases, such as thrombosis, autoimmunity diseases and cancer (17,18).
Therefore, we hypothesized preoperative peripheral WBC count is positively correlated with tumor-infiltrating NETs and predicts a worse outcome in patients receiving esophagectomy. We aimed to investigate the correlation between peripheral WBC count and the prognosis in patients receiving esophagectomy, and explored the potential link between peripheral WBC count and tumor-infiltrating NETs in EC.
Methods
Study population
This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (FUSCC), China. From January 2013 to December 2017, patients undergoing esophagectomy for EC with complete clinical tumor characteristic data, OS records and disease-free survival (DFS) records were enrolled in this retrospective cohort. The exclusion criteria included previous history of cancer, chronic inflammatory diseases and infections including autoimmune diseases, received preoperative neoadjuvant chemotherapy, preoperative and postoperative radiotherapy, received anti-inflammatory drugs or immunosuppressants before surgery, died due to postoperative complications, and a loss of contact during follow-up. For post-operation chemotherapy, was selected according to tumor stage, physician opinion, hospital practice, and patient desire or economic conditions (19). The effectiveness of postoperative chemotherapy was determined basing on computed tomography (CT) scan, positron emission tomography (PET)/CT scan, upper endoscopy, and serum carcinoembryonic antigen levels comprehensively (20,21).
Data were collected from the database of the FUSCC clinical information system. The medical information of each patient was reviewed and recorded, including demographic information, medical history, primary diagnosis, preoperative routine examinations, operative and anesthesia details, tumor differentiation and pathological staging, and DFS and OS time. The preoperative peripheral WBC count was derived from the preoperative routine examination data. The primary outcomes of were DFS and OS. DFS was defined as the length of time from the date of surgery to the date of the first evidence of tumor recurrence or to December 31, 2018. OS was defined as the length of time from the date of surgery to the date of death or the last follow-up date.
Immunohistochemistry (IHC)
Immunofluorescence staining was performed using a horseradish peroxidase method. We simulated the method of selecting samples in Tissue Microarray (TMA) Construction (22), and selected 126 unselected, non-consecutive patients for IHC to explore the level of NETs. Anti-Histone H3 (diluted 1:100, citrulline R2+R8+R17; Abcam 5103, Abcam, Cambridge, MA, USA) and anti-myeloperoxidase (diluted 1:100; Abcam 134132, Abcam) was used. Secondary antibodies with Alexa Flour 555 (1:200, Life Technologies a21432) and Alexa Flour 488 (1:200, Life Technologies a21206) were used, the slides were mounted with Fluoromount-G (Yeasen Biotechnology) with 1 µg/mL DAPI (Beyotime Biotechnology). To minimize the effects of tumor heterogeneity, the number of positive cells per field was estimated using Image Pro plus 6.0 (Media Cybernetics Inc., Bethesda, MD, USA). Two pathologists who were blinded to patients’ information evaluated tumor specimens. The NETs score of IHC was evaluated by two pathologists who were blinded to the patients’ information and was read in a semi-quantitative manner. The NETs score was determined by both the intensity and the extent of staining. The staining intensity was scored as 0 (negative), 1 (weak), 2 (medium) or 3 (strong). The staining extent was scored as 0 (<5%), 1 (5–25%), 2 (26–45%), 3 (46–70%), 4 (71–95%) and 5 (>95%) according to the percentage of the positive staining area in relation to the whole carcinoma area. The final NETs score is the sum of the two pathologists’ respective scores. The IHC score of NETs ranged from 0 to 16 points, and we define 0–8 points as “low” and 9–16 points as “high”. The best cut-off value for IHC and survival prognosis was calculated using X-tile 3.6.1 software (Yale University School of Medicine, New Haven, CT, USA).
Statistical analysis
All sample data are expressed as numerical values or percentages. Independent t-tests were used to compare the WBC count as a continuous variable. WBC cut-off points were generated and analyzed using X-tile 3.6.1 software (Yale University, New Haven, CT, USA), which identified the cut-off with the minimum P values from log-rank ×2 statistics for survival (23). Chi-square tests were used to compare the baseline characteristics of the basic clinical information of patients. Survival curves were generated and evaluated using the Kaplan-Meier method for prognostic factors. Survival differences were compared using the log-rank (Mantel-Cox) test. Cox regression models were built for the analysis of risk factors for survival outcomes in EC patients. Multivariate analyses with a Cox proportional hazards model were used to test independence, significance, and hazard discrimination. Receiver operating characteristic (ROC) curve analysis was performed to determine the efficacy of TNM staging combined with WBC analysis for survival prognosis. R version 3.4.3 was used to perform the nomogram analysis and to generate the calibration chart. Spearman analysis was used for the pairwise analysis of continuous variables and to examine the relationship between peripheral WBC count and tumor-infiltrating NETs. A P value <0.05 was considered statistically significant.
Results
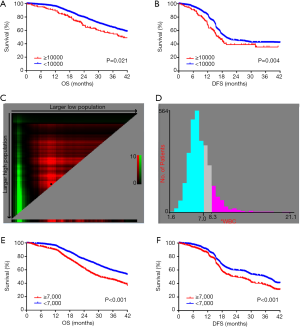
A total of 3,096 consecutive EC patients undergoing esophagectomy were enrolled in this study. Traditionally, leukocytosis is defined as a WBC count of more than 10,000/µL. According to this definition, leukocytosis was observed in 18.3% (568 out of 3,096 patients). To assess the association between preoperative leukocytosis and the prognosis, we performed Kaplan-Meier survival analysis for OS and DFS. The OS and DFS in the leukocytosis group were shorter than in the non-leukocytosis group (Figure 1A,B; P=0.021, P=0.004, respectively). X-tile software was used for the entire cohort to identify the optimal cut-off value for the preoperative WBC count for prognosis, which was 7,000/µL (Figure 1C,D). Based on this WBC cut-off value, the patient characteristics are shown in Table 1. The incidence of moderate leukocytosis was 30.9% (708 out of 3,096 patients). As a continuous variable, moderate leukocytosis was correlated with male sex (P=0.006), advanced T stage (P<0.001), TNM stage (P<0.001) and no postoperative chemotherapy (P<0.001). Kaplan-Meier survival analysis showed that OS and DFS were significantly shorter in the moderate leukocytosis group than in the non-leukocytosis group (Figure 1E,F; P<0.001, respectively).
Full table
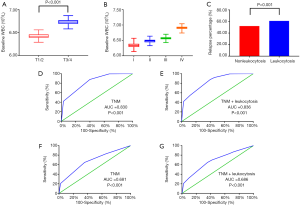
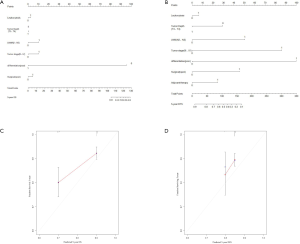
Regarding the correlation between the WBC count and clinical pathology characteristics, advanced T stage (P<0.001), TNM stage (P<0.001) and relapse percentage (P<0.001) were significantly associated with an elevated WBC count (>7,000/µL) (Figure 2A,B,C). To improve the prognostic accuracy of the current TNM staging system, we established a predictive model for EC by combining the TNM staging system and leukocytosis. The combination of both factors achieved the highest area under the curve (AUC) value (0.836 and 0.697), while the AUC predicting OS and DFS based on the only TNM staging system was 0.830 and 0.685, respectively (Figure 2D,E,F,G).
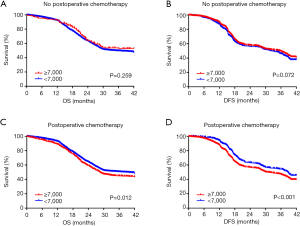
It has been reported that the systemic inflammatory state has a significant effect on the responsiveness and effectiveness of chemotherapy. Therefore, we sought to evaluate the effect of paclitaxel plus cisplatin-based chemotherapy on OS and DFS in EC patients with moderate leukocytosis. For patients who did not receive postoperative chemotherapy, there was no significant association between the moderate leukocytosis status and OS and DFS (Figure 3A,B). However, for patients who received postoperative chemotherapy, OS and DFS were significantly shorter in patients with moderate leukocytosis than in patients in the non-leukocytosis group (Figure 3C,D). To visualize the prognostic efficacy of the WBC count in patients with EC, we created a nomogram of OS and DFS based on the moderate leukocytosis status and other factors that are commonly recognized for prognosis. When the predictor of moderate leukocytosis was included, the prediction accuracy of the nomogram was higher and closer to the reference line of a perfect prediction model (Figure S1A,B,C,D).
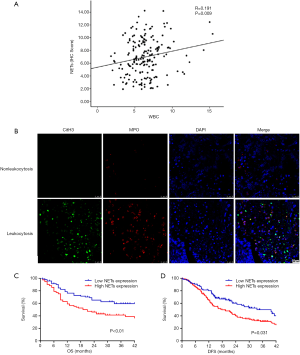
To investigate the association between moderate leukocytosis and tumor-infiltrating NETs, we examined NETs infiltration in tumor specimens of 126 EC patients in FUSCC by immunohistochemistry. Regarding the correlation of the WBC count and NETS infiltration, patients with preoperative moderate leukocytosis had a significantly higher level of NETs infiltration (Figure 4A) than patients without moderate leukocytosis. Representative images are shown in Figure 4B. Kaplan-Meier analysis showed that higher NETs infiltration was associated with poor prognosis in patients with EC (Figure 4C,D, P<0.05, respectively).
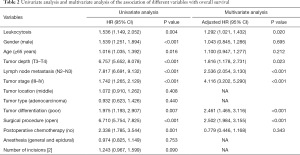
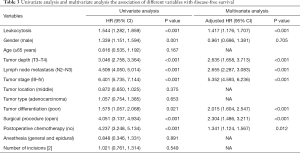
Univariate Cox regression analysis was used to evaluate the clinical characteristics that were significantly associated with OS (Table 2), such as moderate leukocytosis, age (≥65 years), T stage, N stage, tumor stage (III and IV), tumor differentiation (poor), surgical procedure (open), and postoperative chemotherapy (no). Moderate leukocytosis was also confirmed as an independent prognostic factor for OS in the multivariate analysis. Further analysis was conducted to investigate the associations with DFS (Table 3). The univariate analysis showed that leukocytosis, T stage, N stage, tumor stage (III-IV), tumor differentiation (poor), surgical procedure (open), and postoperative chemotherapy (no) were significantly associated with worse DFS. Multivariate analysis that included those factors also showed that moderate leukocytosis was an independent predictor of DFS.
Full table
Full table
Discussion
In this study, we validated that preoperative leukocytosis is a prognostic factor predicting survival and response to postoperative chemotherapy in patients with EC and investigated the prognostic effect of the preoperative WBC count in the context of intra-tumoral NETs infiltration. Our study confirmed that patients with a moderately elevated WBC count based on a cut-off value of 7,000/µL had significantly higher levels of NETs infiltration and were independently associated with worse OS and DFS across the disease stages in this study.
Inflammation is associated with the development and malignant progression of most cancers (24,25). Inflammation is involved in carcinogenesis, tumor growth and metastasis, and the inflammatory microenvironment also contributes to the profound immunosuppression of potential antitumor immune functions, leading to an increasing risk of relapse (26,27). Previous study has reported that the ratio of neutrophils-to-lymphocytes (NLR) and platelet-to-lymphocyte (PLR) can both well-predict the prognosis of EC and the effectiveness of neoadjuvant chemotherapy (28,29). However, there are few related studies about the WBCs count and prognosis of EC. In clinical practice, leukocytosis is often overlooked after infectious and hematologic disease is ruled out, particularly in patients with solid tumors. Recently, owing to the reproducible and convenient nature of hematological measurements, great interest has been generated in elucidating their role in predicting cancer recurrence and death. Currently, the association between higher peripheral blood inflammatory markers and survival has been suggested in various malignancies (30-32). Some studies have also shown that preoperative leukocytosis has a worse prognosis in gastric cancer, colorectal cancer, anal carcinoma and endometrial carcinoma (33-36). In this study, we found that higher preoperative WBC levels were associated with worse OS in EC, which was consistent with previous study (37). Furthermore, a moderate leukocytosis was also associated with worse OS and DFS. Univariate analysis also demonstrated that preoperative moderate leukocytosis was a significant risk factor, and multivariate analysis demonstrated that preoperative moderate leukocytosis was an independent prognostic marker for poor prognosis in EC.
Clinically, the TNM staging system is considered the gold standard for staging in EC, however, this staging system is based almost exclusively on the anatomical spread of cancer and narrowly examines the tumor cells without considering the effects of the host immune response (38,39). In this study, we found that a new predictive model combining tumor-associated leukocytosis and the TNM staging system had better prognostic accuracy for EC than the TNM staging system alone. In addition, the TNM staging system does not predict the response to chemotherapy, while the increased levels of inflammatory cells were showed be associated with the efficacy of chemotherapy (39,40). Paclitaxel plus cisplatin-based chemotherapy is one of the main options for patients after esophagectomy (41). We observed that in patients who received postoperative chemotherapy, and with a moderate leukocytosis were likely to have a poor OS and DFS, while there was no correlation found for patients who did not receive chemotherapy. These findings may suggest that EC patients with postoperative chemotherapy and moderate leukocytosis need more attention and more choices of adjuvants therapies.
Neutrophils are the most abundant type of peripheral leukocyte and are capable of destroying invading microorganisms, through phagocytosis and intracellular degradation, release of granules (15). In addition, neutrophils could infiltrate many types of tumors and form NETs (42,43). Many studies have shown that the dysregulation of neutrophil activation leads to the overproduction of NETs and is positively associated with poor prognosis in many diseases, such as sepsis, wound healing, autoimmune disease and thrombosis (17,44). In recent years, an increasing number of studies has shown that NETs are involved in tumor progression and metastasis (45,46). In this study, we found that preoperative moderate leukocytosis was significantly associated with higher levels of NET infiltration, and higher levels of NETs were also associated with poor prognosis in patients with EC. These findings suggest a possible mechanism for the adverse prognosis of leukocytosis.
Our study has several limitations. This study was retrospective and was not randomized. Patients were recruited from a single center. The association between moderate leukocytosis and the poor OS and DFS in patients receiving postoperative chemotherapy was based on subgroup analysis. Although we found that moderate leukocytosis and higher levels of NET infiltration were involved in tumor progression, the underlying mechanisms still need further investigation.
In conclusion, the preoperative moderate leukocytosis was negatively correlated with survival in EC. The inclusion of the WBC count in the TNM staging system could provide better prognostic information for risk stratification. Additionally, the correlation between moderate leukocytosis and intra-tumoral NETs infiltration provided a potential mechanism for the adverse impact of preoperative leukocytosis.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (No. 81873948, 81871591), National Key R&D Program of China (No. 2018YFC2001900-04), Shanghai Shenkang Hospital Development Center Clinical Science and Technology Innovation Project (No. SHDC12018105).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.190). All authors report grants from The National Natural Science Foundation of China, grants from The National Natural Science Foundation of China, grants from The National Key R&D Program of China, grants from Shanghai Shenkang Hospital Development Center Clinical Science and Technology Innovation Project, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (FUSCC), China.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [Crossref] [PubMed]
- Malhotra GK, Yanala U, Ravipati A, et al. Global trends in esophageal cancer. J Surg Oncol 2017;115:564-79. [Crossref] [PubMed]
- Koshy M, Esiashvilli N, Landry JC, et al. Multiple management modalities in esophageal cancer: combined modality management approaches. Oncologist 2004;9:147-59. [Crossref] [PubMed]
- Lou F, Sima CS, Adusumilli PS, et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol 2013;8:1558-62. [Crossref] [PubMed]
- Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 2014;6 Suppl 3:S289-97. [PubMed]
- Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol 2007;25:507-12. [Crossref] [PubMed]
- Talsma K, van Hagen P, Grotenhuis BA, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol 2012;19:2142-8.
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Lin EW, Karakasheva TA, Hicks PD, et al. The tumor microenvironment in esophageal cancer. Oncogene 2016;35:5337-49. [Crossref] [PubMed]
- Afghahi A, Purington N, Han SS, et al. Higher Absolute Lymphocyte Counts Predict Lower Mortality from Early-Stage Triple-Negative Breast Cancer. Clin Cancer Res 2018;24:2851-8. [Crossref] [PubMed]
- de Nonneville A, Barbolosi D, Andriantsoa M, et al. Validation of Neutrophil Count as An Algorithm-Based Predictive Factor of Progression-Free Survival in Patients with Metastatic Soft Tissue Sarcomas Treated with Trabectedin. Cancers (Basel) 2019. [Crossref] [PubMed]
- Mabuchi S, Matsumoto Y, Isohashi F, et al. Pretreatment leukocytosis is an indicator of poor prognosis in patients with cervical cancer. Gynecol Oncol 2011;122:25-32. [Crossref] [PubMed]
- Gouw ZA, Paul DBJ, Navran A, et al. Baseline peripheral blood leukocytosis: Biological marker predicts outcome in oropharyngeal cancer, regardless of HPV-status. Oral Oncol 2018;78:200-6. [Crossref] [PubMed]
- Abdel-Latif MM, Duggan S, Reynolds JV, et al. Inflammation and esophageal carcinogenesis. Curr Opin Pharmacol 2009;9:396-404. [Crossref] [PubMed]
- Rosales C.. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol 2018;9:113. [Crossref] [PubMed]
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532-5. [Crossref] [PubMed]
- Papayannopoulos V.. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018;18:134-47. [Crossref] [PubMed]
- Ravindran M, Khan MA, Palaniyar N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019. [Crossref] [PubMed]
- Li Y, Zhao W, Ni J, et al. Predicting the Value of Adjuvant Therapy in Esophageal Squamous Cell Carcinoma by Combining the Total Number of Examined Lymph Nodes with the Positive Lymph Node Ratio. Ann Surg Oncol 2019;26:2367-74. [Crossref] [PubMed]
- Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616-23. [Crossref] [PubMed]
- Abate E, DeMeester SR, Zehetner J, et al. Recurrence after esophagectomy for adenocarcinoma: defining optimal follow-up intervals and testing. J Am Coll Surg 2010;210:428-35. [Crossref] [PubMed]
- Hu X, Li YQ, Li QG, et al. ITGAE Defines CD8+ Tumor-Infiltrating Lymphocytes Predicting a better Prognostic Survival in Colorectal Cancer. Ebiomedicine 2018;35:178-88. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 2018;32:1267-84. [Crossref] [PubMed]
- Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019;51:27-41. [Crossref] [PubMed]
- Nakamura K, Yoshida N, Baba Y, et al. Elevated preoperative neutrophil-to-lymphocytes ratio predicts poor prognosis after esophagectomy in T1 esophageal cancer. Int J Clin Oncol 2017;22:469-75. [Crossref] [PubMed]
- McLaren PJ, Bronson NW, Hart KD, et al. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios can Predict Treatment Response to Neoadjuvant Therapy in Esophageal Cancer. J Gastrointest Surg 2017;21:607-13. [Crossref] [PubMed]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237-49. [Crossref] [PubMed]
- Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer 2015;121:545-55. [Crossref] [PubMed]
- Urakawa S, Yamasaki M, Goto K, et al. Peri-operative monocyte count is a marker of poor prognosis in gastric cancer: increased monocytes are a characteristic of myeloid-derived suppressor cells. Cancer Immunol Immunother 2019;68:1341-50. [Crossref] [PubMed]
- Wang YL, Ge XX, Wang Y, et al. The values of applying classification and counts of white blood cells to the prognostic evaluation of resectable gastric cancers. BMC Gastroenterol 2018;18:99. [Crossref] [PubMed]
- Hu X, Li YQ, Li QG, et al. Baseline Peripheral Blood Leukocytosis Is Negatively Correlated With T-Cell Infiltration Predicting Worse Outcome in Colorectal Cancers. Front Immunol 2018;9:2354. [Crossref] [PubMed]
- Schernberg A, Huguet F, Moureau-Zabotto L, et al. External validation of leukocytosis and neutrophilia as a prognostic marker in anal carcinoma treated with definitive chemoradiation. Radiother Oncol 2017;124:110-7. [Crossref] [PubMed]
- Worley MJ, Nitschmann CC, Shoni M, et al. The significance of preoperative leukocytosis in endometrial carcinoma. Gynecol Oncol 2012;125:561-5. [Crossref] [PubMed]
- Chen XF, Qian J, Pei D, et al. Prognostic value of perioperative leukocyte count in resectable gastric cancer. World J Gastroenterol 2016;22:2818-27. [Crossref] [PubMed]
- Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 2005;5:845-56. [Crossref] [PubMed]
- Hu X, Li YQ, Ma XJ, et al. A Risk Signature With Inflammatory and T Immune Cells Infiltration in Colorectal Cancer Predicting Distant Metastases and Efficiency of Chemotherapy. Front Oncol 2019;9:704. [Crossref] [PubMed]
- Kirilovsky A, Marliot F, El SC, et al. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol 2016;28:373-82. [Crossref] [PubMed]
- Ai D, Chen Y, Liu Q, et al. Comparison of paclitaxel in combination with cisplatin (TP), carboplatin (TC) or fluorouracil (TF) concurrent with radiotherapy for patients with local advanced oesophageal squamous cell carcinoma: a three-arm phase III randomized trial (ESO-Shanghai 2). BMJ Open 2018;8:e020785. [Crossref] [PubMed]
- Treffers LW, Hiemstra IH, Kuijpers TW, et al. Neutrophils in cancer. Immunol Rev 2016;273:312-28. [Crossref] [PubMed]
- Wu L, Saxena S, Awaji M, et al. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers (Basel) 2019. [Crossref] [PubMed]
- Yang H, Biermann MH, Brauner JM, et al. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front Immunol 2016;7:302. [Crossref] [PubMed]
- Erpenbeck L, Schon MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene 2017;36:2483-90. [Crossref] [PubMed]
- Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018;361:6409. [Crossref] [PubMed]


