Investigation of nicotinamide as more than an anti-phosphorus drug in chronic hemodialysis patients: a single-center, double-blind, randomized, placebo-controlled trial
Introduction
Hyperphosphatemia is a common complication of late-stage chronic kidney disease (CKD) (1). Many studies have revealed the association between hyperphosphatemia and increased mortality, but no randomized control trials have confirmed the effect of a lower serum phosphorus level on reduction of all-cause death. Although several strategies such as dietary phosphate restriction, oral phosphate binders (including calcium-based phosphorus binders and non-calcium phosphorus binders), and increased dialysis time or frequency have been adopted to counter hyperphosphatasemia, the improvement of clinical outcomes is far from satisfactory (2). Hard endpoints such as cardiovascular events and overall mortalities remain extremely higher in patients with end-stage renal disease (ESRD) (3). These results are a reminder that the sole focus on serum phosphorus level to decrease cardiovascular events and overall mortalities may not be sufficient in patients with ESRD.
Several hormones and growth factors, including the parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), α-Klotho, and 25-hydroxyvitamin D (25-OH vitamin D), play critical roles in maintaining the balance of phosphorus (4). It remains controversial if the FGF23 directly participates the progression of vascular calcification (5). Several studies have shown the correlation between FGF23 and calcium accumulation in the arteries among patients with chronic dialysis (6), but the majority of researchers believe that FGF23’s effects are primarily related to left ventricular hypertrophy (LVH) and heart failure rather than calcification (7). In contrast, mounting evidence indicates the well-supported protective role for α-Klotho, the obligatory co-receptor for FGF23, on vascular calcification (8). Thus, the effect of anti-phosphorus drugs on FGF23 and Klotho change has become of great concern in clinical practice.
Nicotinamide (NAM), or niacinamide, is a water-soluble vitamin that is part of the vitamin B complex (9). Recently, NAM was found to act as phosphate-reducing agent in human and animal studies by inhibiting intestinal Na-Pi2b cotransporter, which conducts the active transportation of phosphate in the intestinal luminal mucosal membrane. Several studies have evaluated its efficacy and safety in reducing serum phosphorus in patients with hemodialysis, and results suggest that it may be a useful pharmacological adjunctive therapy to the binder-based approaches (10). Few studies have investigated the effect of NAM on the regulation of FGF23 and Klotho. Ix et al. evaluated the efficacy of lanthanum carbonate (LC) and/or NAM in stage 3b/4 CKD patients. They found no significant effect of LC and/or NAM treatment on lowering serum phosphate or FGF23 in stage 3b/4 CKD over 12 months (11). Another open-labeled study designed to examine the non-inferiority of NAM to sevelamer (SEV) in patients with chronic hemodialysis found no significant change of FGF23 in the NAM group (4). However, the high dropout rate of this trial weakens the reliability of its conclusion. More randomized, double-blind, controlled trials are warranted to investigate the effect of NAM on FGF23 and Klotho in patients with chronic hemodialysis.
Therefore, we conducted this double-blind, randomized, placebo-controlled trial to evaluate the effect of NAM on serum phosphorus and the main factors that contribute to phosphate regulation in patients with hemodialysis. The primary objective was to evaluate the efficacy of NAM in controlling hyperphosphatasemia. The secondary objectives were to evaluate the change of serum FGF23 and Klotho in NAM-treated patients. Furthermore, we evaluated the effect of NAM on abdominal aortic calcification (AAC), which had a good correlation with coronary calcification according to the Kauppila score (KS) based on an X-ray of the abdomen (12).
Methods
Study design and ethical statements
This study was a single-center, double-blind, randomized, placebo-controlled trial with a duration of 52 weeks. The study was conducted in compliance with the protocols and principles of the Declaration of Helsinki and approved by the Clinical Research Ethical Committee of Tianjin Haihe Hospital (approval number: 2017-kt-07). All participants were informed in detail about the benefits and risks, and written consent was obtained before enrollment.
Study population and criteria
The total target sample size was 74 subjects (37 subjects per treatment arm). With this sample size, a difference of 0.2 mmol/L between the NAM group and placebo group in serum phosphorus could be detected with 80% power assuming a standard deviation of 0.43 at a significance level of 95%. We anticipated a dropout rate of no more than 20%, thus necessitating the sample size increase to 94 subjects. We screened 268 patients who accepted regular hemodialysis in Tianjin Haihe Hospital between June 2017 and June 2018. In total, 98 patients who met the following criteria were recruited: (I) patients who received hemodialysis treatment 3 times per week and took calcium (carbonate or acetate) regularly as the phosphate binder; and (II) patients with a stable clinical condition with serum phosphorus levels ≥1.6 mmol/L, serum total calcium levels ≤2.37 mmol/L, and serum intact PTH levels between 60 and 800 pg/mL. The exclusion criteria were the following: (I) patients younger than 18 years of age at the initiation of hemodialysis; (II) patients with abnormal liver function; (III) patients who received SEV, lanthanum, or other non-calcium phosphorus binders; (IV) patients with ongoing chemotherapy and body weight loss >3 kg in the previous 3 months; and (V) patients who were either unlikely to survive or planned to have kidney transplant or transferred to another renal center within 6 months.
Randomization and intervention
Patients were randomly assigned in a 1:1 ratio to receive either NAM or a placebo in a double-blind manner for 52 weeks. A random sequence was generated by a computer and coded. Opaque, sealed envelopes were used to conceal the allocation. Placebo tablets (purchased from Shanghai Yurui Biotechnology Pharmaceutical Co., Ltd., Anyang, China) with an identical appearance, flavor, and package of NAM were administered to patients in the placebo group. Study members, including the designer and the coordinator, remained blinded to the treatment allocation. Patients in the NAM group were initiated on NAM at a dose of 500 mg/day (1 tablet after meal), with an up-titration by 500 mg/day every 2 weeks until the dosage reached 1,500 mg/day (3 times/day, 1 tablet/time). The placebo was administered with the exact same dosage as the NAM group. In both groups, calcium carbonate or acetate therapy was continued at a dose of 500 mg, 1–3 times daily with a 4-week up-titrated period as clinically required.
Data collection
Demographic and baseline clinical data including age, sex, height, body weight, body mass index, systolic blood pressure, diastolic blood pressure, primary disease, duration of hemodialysis, vitamin D therapy, and phosphate binder therapy were collected at the start of study enrollment. Comorbid conditions were investigated and included a comprehensive list of diseases, including coronary artery disease, heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes with or without complications, hemiplegia, leukemia, solid tumor, malignancy, and AIDS. The Charlson Comorbidity Index was adopted to score the comorbid conditions.
Treatment, laboratory analyses, and imaging studies
In this 52-week clinical trial, all patients were treated with regular hemodialysis 3 times per week with a calcium concentration of 1.25% and followed up at weeks −4, 0, 2, 4, 8, 12, 24, and 52. All enrolled patients received adequate diet and lifestyle counseling. Serum phosphorus, total calcium, and other biochemical indexes were assayed in an on-site biochemistry laboratory using standard auto-analyzer techniques (Modular IIP system; Roche Diagnostics, Basel, Switzerland). The 25-OH vitamin D level was tested by Roche Cobas601 electrochemiluminescence immunoassay (Roche Diagnostics), and serum intact PTH (iPTH) was determined by a chemiluminometric immunoassay (Roche Diagnostics) every 4 weeks. Serum FGF23 and Klotho levels were measured using enzyme-linked immunosorbent assay kits according to instructions (Biotopped, Beijing, China, FGF23:intra-assay coefficient of variation: <10%, inter-assay coefficient of variation: <12%; detection limit of the assay: 5 ng/L; Klotho: intra-assay coefficient of variation: <10%, inter-assay coefficient of variation: <12%; detection limit of the assay: 10 ng/L) at weeks 0, 4, 8, 12, 24, and 52. All the serum specimen was centrifuged and then immediately frozen at −80 °C prior to analysis at the end of the study in a central laboratory and all the measurements were made on the first thaw. The AAC was evaluated by lateral abdominal radiography from vertebra T10 to the first 2 vertebrae of the sacrum (13). The X-rays were evaluated by 2 independent radiology experts (LS and ZHX) who did not have access to the patients’ clinical data. According to KS, AAC from L1 to L4 was scored independently from 0 to 3 points (0 points, no calcification; 1 point, minor calcification: the range of calcification was less than 1/3 of the corresponding aorta; 2 points, moderate calcification: the range of calcification was more than 1/3 and less than 2/3 of the corresponding aorta; 3 points, severe calcification: the range of calcification was more than 2/3 of the corresponding aorta). Each patient’s ACC was scored from 0 to 24 points. Prescriptions of concomitant medications that could have had a direct effect on serum phosphorus levels such as vitamin D and cinacalcet were left unchanged as much as possible in accordance with standard clinical practice. Other routine treatments such as antihypertensive drugs and erythropoietin were administered according to the patient’s clinical needs. Trial medication tablets were counted by the investigators monthly to calculate compliance. Several parameters such as body weight, systolic blood pressure, diastolic blood pressure, presence of edema (scored from 0 to 3+), cardiovascular events, and the number and reason for hospitalization were recorded during the follow-up visits at weeks 0, 2, 4, 8, 12, 24, and 52.
Statistical analysis
The placebo group and NAM group were compared in terms of patients’ demographic and baseline clinical characteristics using the t-test for continuous variables and chi-square test for categorical variables. We compared the phosphorus levels at each visit time to depict the variation curve. An independent t-test was used to compare the differences of serum phosphorus levels between the two groups from 0 to 52 weeks. We adopted the intention-to-treat analysis to reduce the effect of dropout, which might have diverged from the random assignment (14). The last observation carried forward method was used to replace missing data to diminish the different attrition rates between two groups when we were investigating the phosphorus level (15). Since the FGF23 and Klotho levels were measured several times, repeated-measure analysis of variance (ANOVA) and linear mixed models (LMMs) were used to compare the overall difference of FGF23 and Klotho levels between the two groups, as we wanted to take advantage of these data. We used the treatment group (NAM or placebo), baseline values, time (0, 4, 8, 12, 24, and 52 weeks), dosage of calcium prescribed as the fixed effects, and patient identification as the random effects. A compound symmetry covariance structure was employed for the repeated factor of time. Continuous variables are expressed as the mean ± standard deviation or median (interquartile range). Count data are presented as n (%). A P value <0.05 was considered statistically significant. All probabilities were two-tailed. Statistical analyses were performed using IBM SPSS Statistics, version 20 for Windows (IBM Corp., Armonk, NY, USA).
Results
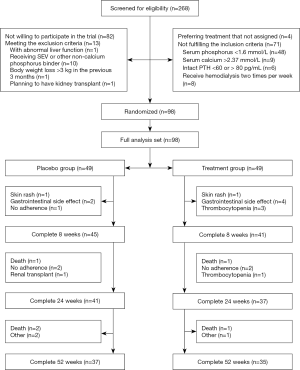
We screened the 268 patients who received regular hemodialysis in our center for eligibility of enrollment; 82 patients were excluded owing to their unwillingness to participate in the trial, 71 patients were ruled out because of their inability to meet the inclusion criteria, and 13 patients met a exclusion criterion. In total, 102 patients were randomly assigned to the NAM or placebo group, and 3 patients withdrew before the trial started owing to their treatment preference. Thus, 49 patients in the placebo group and 49 patients in the NAM group comprised the full-analysis set, and 37 patients in the placebo group and 35 patients in the NAM group completed the 52-week follow-up. Specifically, 12 patients in the placebo group dropped out because of death (n=3), renal transplant (n=1), gastrointestinal side effect (n=2), skin rash (n=1), no adherence to treatment (n=3), and other (n=2), and 14 patients in the NAM group withdrew because of death (n=2), gastrointestinal side effect (n=4), thrombocytopenia (n=4), skin rash (n=1), no adherence to treatment (n=2), and other (n=1). A flow chart of the patients and reasons for withdrawal from the study is shown in Figure 1.
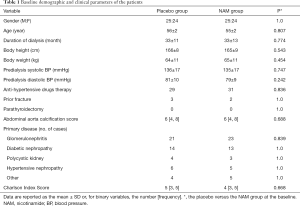
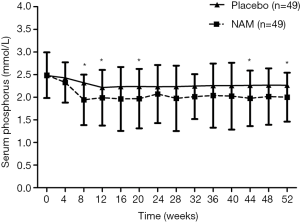
The demographic and clinical parameters between the two groups were well balanced (Table 1), and all baseline biochemical characteristics between the groups were comparable (Table 2). Serum phosphorus levels during the follow-up are summarized in Figure 2. In the placebo group, there was a gradual decrease in the serum phosphorus level from 2.48±0.50 to 2.32±0.42 mmol/L in the first 8 weeks (P<0.01), which reached its lowest value of 2.22±0.49 mmol/L at week 12, and then the serum phosphorus level fluctuated around this level for the rest of the follow-up. In the NAM group, the serum phosphorus level decreased more dramatically from 2.49±0.50 to 1.94±0.56 mmol/L and reached its lowest value at week 8. Although it increased and fluctuated afterward, the serum phosphorus level remained significantly lower in the NAM group than in the placebo group in weeks 8 (2.32±0.42 vs. 1.94±0.56 mmol/L, P=0.000), 12 (2.22±0.49 vs. 1.99±0.61 mmol/L, P=0.046), 20 (2.24±0.67 vs. 1.97±0.66 mmol/L, P=0.048), 44 (2.26±0.73 vs. 1.97±0.61 mmol/L, P=0.037), and 52 (2.26±0.66 vs. 2.00±0.54 mmol/L, P=0.032). We also performed an analysis of phosphorus levels which only included the patients who completed the 52 weeks follow-up, and there was no significant difference between the two groups Table S1.
Full table
Full table

Full table
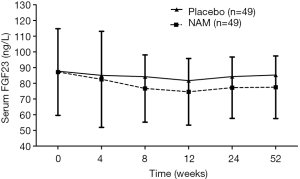
We compared the change of serum FGF23 levels in both groups. The variation curve showed a gradual decrease of the FGF23 level in the placebo group during the first 8 weeks from 87.70±30.34 to 84.19±26.35 ng/L, which reached its lowest value of 81.73±20.38 ng/L at week 12. Then, the FGF23 level increased slowly and reached 85.31±20.09 ng/L; thus, it almost returned to its baseline level at the end of the trial. The serum FGF23 level decreased more obviously in the NAM group than in the placebo group, although no significant difference was detected by the independent t-test at each time between the groups. When LMMs for repeated-measure ANOVA were introduced to conduct the overall comparisons, a significant effect of NAM on the FGF23 level was found (F=4.766, P=0.032). No significant interaction of group × time in FGF23 change was detected in our trial (F=0.280, P=0.891). The baseline FGF23 level also had significant effects on changes of the FGF23 level (F=100.559, P=0.000), but not on the time and calcium dosage (F=1.550, P=0.187 and F=0.251, P=0.617, respectively). Changes of the serum FGF23 level during the follow-up are summarized in Figure 3.
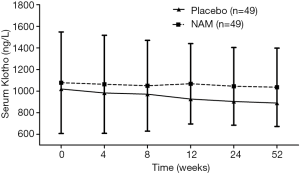
We also investigated the difference of changes in the serum Klotho level between the two groups. During the 52-week follow-up, a downward trend of the serum Klotho level was found in most participants, although a significant difference was not detected between every 2 points in both groups. When we compared the serum Klotho level between the groups, no significant difference was found at each time either (Figure 4). However, when we adopted the LMMs for overall comparisons by repeated-measure ANOVA, a significant effect of NAM on the serum Klotho level was revealed (F=5.481, P=0.021); thus, NAM significantly slowed the decreasing rate of the serum Klotho level in patients with dialysis. The baseline serum Klotho level also had a significant effect on changes in the Klotho level (F=174.070, P=0.000). No significant effect of time, interaction of group × time, and calcium dosage was detected in our trial (F=0.290, P=0.885; F=0.342, P=0.849; and F=0.001, P=0.970, respectively).
Since the FGF23/Klotho axis has been implicated in mediating vascular calcification and LVH, we also evaluated the effect of NAM on AAC. Given that the KSs were not distributed normally, the nonparametric test was adopted. At baseline, the KSs between the placebo and NAM groups were balanced [6 (Jeny4, 8) and 6 (Jeny4, 8), respectively; P=0.688]. After the 52-week follow-up, no significant difference of the KS was detected between baseline and the end of the study in both groups [placebo group: from 6 (Jeny4, 8) to 6 (Jeny5, 9), P=0.217; NAM group: from 6 (Jeny4, 8) to 6 (Jeny4, 9), P=0.536], and there was no significant difference between the two groups (P=0.805).
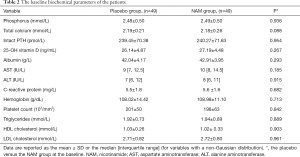
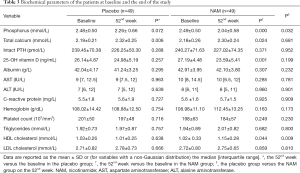
Biochemical parameters such as calcium, 25-OH vitamin D, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and C-reactive protein (CRP) levels were also investigated throughout the 52-week follow-up. The serum total calcium concentration increased in both groups (placebo group: from 2.19±0.21 to 2.32±0.25 mmol/L, P=0.006; NAM group: from 2.18±0.26 to 2.30±0.24 mmol/L, P=0.024), and there was no significant difference between the groups at the end of this trial (P=0.681). A downward trend of the 25-OH vitamin D level was detected in both groups, but only the change in the NAM group was significantly different (placebo group: from 26.14±4.87 to 24.98±5.19 ng/mL, P=0.257; NAM group: from 27.19±4.48 to 23.59±5.41 ng/mL, P=0.001). The change of the iPTH level from baseline to the end of the study did not reach a significant difference in either group (placebo group: from 239.45±70.38 to 226.25±50.30 pg/mL, P=0.288; NAM group: from 240.27±71.63 to 227.02±74.35 pg/mL, P=0.371), and there was no significant difference between the two groups (P=0.952). We also compared the serum HDL-C, LDL-C, CRP, aspartate aminotransferase, alanine aminotransferase, and albumin changes between the two groups: a significant increase of HDL-C (from 1.02±0.33 to 1.15±0.28 mmol/L, P=0.044) was found in the NAM group, and there was no significant difference between the groups in the other biochemical parameters (Table 3).
Full table
We observed an increased rate of thrombocytopenia (placebo group: 0%; NAM group: 8%) in the NAM group. Among the 4 cases of thrombocytopenia, 3 cases were detected in the first 8 weeks. The mean NAM dosage was 1,250 mg/day, and the lowest platelet count came up to 75,000/mm3. Gastrointestinal side effect was another reason participant in the NAM group to withdrawal from the study. In the NAM group, 2 patients suffered from nausea and 2 patients suffered diarrhea in the first 2 weeks (1 patient experienced vomiting and 1 patient developed nausea in the placebo group). It should be noted that patients rapidly recovered from the gastrointestinal side effects and the thrombocytopenia in all cases after withdrawal of NAM within 3 weeks. No participant experienced serious adverse events during the 52-week follow-up. Finally, 3 patients in the placebo group and 2 patients in NAM group accepted hospitalization therapy (placebo: 1 for heart failure and 2 for hypertension; NAM: 1 for pneumonia and 1 for hypertension).
Discussion
Hyperphosphatemia is a common complication of late-stage CKD, which is highly correlated with the cardiovascular events and overall mortalities (16). In this 52-week clinical trial, both placebo and NAM groups underwent a gradual decrease of the serum phosphorus level in the first 4 weeks. We attributed this change to the lifestyle consultant and up-dosage of calcium-based phosphorus binders. In the remaining follow-up time, patients in the NAM group experienced a more rapid decrease of the serum phosphorus level, which reached its lowest value at week 8. Although the serum phosphorus level fluctuated above this value for the remainder of the follow-up, it remained significantly lower than that of the placebo group at weeks 8, 12, 20, 44, and 52. Our findings confirmed that, as an add-on therapy, NAM can further alleviate hyperphosphatemia in patients with hemodialysis. Even though the incidence rate of adverse events (e.g., gastrointestinal side effects and thrombocytopenia) were higher in the NAM group than in the placebo group, these problems rapidly resolved when NAM was withdrawn; these side-effect findings were similar to those found in Ix’s studies (11).
As a critical modulator of serum phosphorus, FGF23 is correlated with calcium accumulation in arteries (17,18), so we also investigated the effect of NAM on change in the serum FGF23 level. We found a downward trend of the FGF23 level in both groups, but the change between every 2 times of the study was not significantly different in either group. Furthermore, no significant difference was detected between the groups by the independent t-test at each time. We attributed the failure to detect a significant difference to the relatively small sample size. This speculation is supported by the fact that when we adopted the LMMs for repeated-measure ANOVA to compare the overall difference of the two groups, a significant effect of NAM on the serum FGF23 level was revealed. Our finding showed that based on the calcium-containing phosphate binders, NAM can further decrease the phosphorus level and reduce the serum FGF23 level in patients with hemodialysis. Similarly, Malhotra et al.’s study found a downward trend of the serum FGF23 level in the niacin-treated group during the first year in CKD patients (19), although this trend diminished by the third year. We also noticed that in Malhotra et al.’s study, the decrease of the serum phosphorus level was miniscule [−0.07 mg/dL (range, −0.13 to −0.01 mg/dL)]. A reasonable explanation for the differences with our study is that in Malhotra et al.’s study, patients with a serum creatinine concentration >2.5 mg/dL were excluded; thus, all enrolled patients in their study had considerable residual renal function to eliminate most of the excess serum phosphate, so niacin had less of an effect on the serum phosphorus level in Malhotra et al.’s study. This explanation is also relevant to the change of the FGF23 level in Malhotra et al.’s study.
Klotho is another critical hormone in phosphorus and bone metabolism (20,21), and it was reported to attenuate the development of myocardial hypertrophy caused by uremic toxins in mice with CKD (22). Mounting evidence has indicated a well-supported protective role for α-Klotho on vascular calcification (23,24). In our study, a gradual decrease of the Klotho level was found in both groups, and this finding is consistent with Lenglet et al.’s study, which found a downward trend of the serum Klotho level in the NAM group (4). Unfortunately, Lenglet et al.’s study only investigated the effect of NAM effect on Klotho by using post-hoc analysis with no placebo/control, and this limitation weakened the reliability of their conclusion. In the present study, we tested the Klotho values in both NAM and placebo groups at multiple times and compared these values by repeated-measure ANOVA. We found that although the serum Klotho level decreased in both groups, a significant effect of NAM on Klotho was revealed; thus, NAM significantly slowed down the decreasing rate of the Klotho level in patients with hemodialysis. Hence, we have adequate reason to speculate that NAM may alleviate cardiovascular calcification by increasing the cardiovascular protective hormone in patients with hemodialysis. To verify our deduction, we evaluated the AAC, which showed good correlation with coronary calcification based on the KS at weeks 0 and 52. However, this finding may be attributable to the short follow-up and small sample size, as no difference was detected between the groups using the Mann-Whitney U test (z=−0.197, P=0.844).
Several features of this study have strengthened the reliability of our findings. First, to our knowledge, this is the first randomized placebo-controlled trial that was designed to investigate the effect of NAM on FGF23 and Klotho levels. The randomization guaranteed the balance of baseline characteristics of all participants and reduced the bias from enrollment. Second, since most previous studies had a short-term follow-up (4,25), the 52-week follow-up period helped us to reveal the mid-term to long-term effect of NAM on the metabolism of phosphorus and its related factors. Third, the adoption of LMMs for repeated-measure ANOVA enabled us to take advantage of the clinical data and enhanced our ability to discover the effect of NAM on the metabolism of phosphorus and its related factors. Lastly, to our knowledge, this is the first study to discuss the effect of NAM on AAC, and the study found a good correlation with coronary calcification, although the observation period was relatively short. More large-scale, long-term prospective studies are warranted.
Moreover, we acknowledge several limitations in the present study. First, this was a single-center study with a small sample size, and these factors weaken the strength of our findings, although the repeated-measure ANOVA increased the sample size. More prospective studies with large samples are needed to further confirm the conclusion. Second, owing to the poor health condition of the enrolled patients, the dropout rate was high (26) although we did adopt the LMMs to take advantage of all the available data. Third, all study participants accepted lifestyle counseling at each visit, but protein intake and nutritional status were not assessed or introduced into the analysis as a covariate, which might have affected the results of the study. Dietary protein intake and nutritional status should be considered in our future studies. At last, it remains unclear that if the NAM’s FGF 23-lowering effect was out of its phosphorus reducing function or some other mechanisms. We will explore this issue in our further studies.
Conclusions
As an add-on therapy to calcium-based phosphate binders, NAM can further decrease the phosphorus level, reduce the FGF23 level, and slow the decreasing rate of the Klotho level; therefore, NAM may alleviate cardiovascular damage in patients with hemodialysis. More large-scale, long-term clinical trials are needed to further evaluate the effect of NAM on vascular calcification in patients with hemodialysis.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (number [no.] 81470187), Natural Science Foundation of Tianjin (no. 18JCYBJC26100), and Scientific Research Funding of Tianjin Medial University Chu Hsien-I Memorial Hospital (grant no.: 2018ZDKF08).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.228). Dr. RX, Dr. LXX, Dr. BS, Dr. SL, and Dr. XYL report grants from National Natural Science Foundation of China (number [no.] 81470187), grants from Natural Science Foundation of Tianjin (no. 18JCYBJC26100), grants from Scientific Research Funding of Tianjin Medial University Chu Hsien-I Memorial Hospital (grant no.: 2018ZDKF08), during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in compliance with the protocols and principles of the Declaration of Helsinki and approved by the Clinical Research Ethical Committee of Tianjin Haihe Hospital (approval number: 2017-kt-07). All participants were informed in detail about the benefits and risks, and written consent was obtained before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cozzolino M, Ciceri P, Galassi A. Hyperphosphatemia: a novel risk factor for mortality in chronic kidney disease. Ann Transl Med 2019;7:55. [Crossref] [PubMed]
- Calvo MS, Sherman RA, Uribarri J. Dietary phosphate and the forgotten kidney patient: a critical need for FDA regulatory action. Am J Kidney Dis 2019;73:542-51. [Crossref] [PubMed]
- Copland M, Komenda P, Weinhandl ED, et al. Intensive hemodialysis, mineral and bone disorder, and phosphate binder use. Am J Kidney Dis 2016;68:S24-32. [Crossref] [PubMed]
- Lenglet A, Liabeuf S, El Esper N, et al. Efficacy and safety of nicotinamide in haemodialysis patients: the NICOREN study. Nephrol Dial Transplant 2017;32:870-9. [Crossref] [PubMed]
- Stöhr R, Schuh A, Heine GH, et al. FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol (Lausanne) 2018;9:351. [Crossref] [PubMed]
- Srivaths PR, Goldstein SL, Silverstein DM, et al. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol 2011;26:945-51. [Crossref] [PubMed]
- Scialla JJ, Lau WL, Reilly MP, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 2013;83:1159-68. [Crossref] [PubMed]
- Yamada S, Giachelli CM. Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 2017;100:87-93. [Crossref] [PubMed]
- Pissios P. Nicotinamide N-methyltransferase: more than a vitamin B3 clearance enzyme. Trends Endocrinol Metab 2017;28:340-53. [Crossref] [PubMed]
- Rennick A, Kalakeche R, Seel L, et al. Nicotinic acid and nicotinamide: a review of their use for hyperphosphatemia in dialysis patients. Pharmacotherapy 2013;33:683-90. [Crossref] [PubMed]
- Ix JH, Isakova T, Larive B, et al. Effects of Nicotinamide and Lanthanum Carbonate on Serum Phosphate and Fibroblast Growth Factor-23 in CKD: The COMBINE Trial. JASN 2019;30:1096-108. [Crossref] [PubMed]
- Bellasi A, Ferramosca E, Muntner P, et al. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int 2006;70:1623-8. [Crossref] [PubMed]
- Kauppila LI, Polak JF, Cupples LA, et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997;132:245-50. [Crossref] [PubMed]
- Sheiner LB, Rubin DB. Intention-to-treat analysis and the goals of clinical trials. Clin Pharmacol Ther 1995;57:6-15. [Crossref] [PubMed]
- Shao J, Jordan DC, Pritchett YL. Baseline observation carry forward: reasoning, properties, and practical issues. J Biopharm Stat 2009;19:672-84. [Crossref] [PubMed]
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011;305:1119-27. [Crossref] [PubMed]
- Takashi Y, Fukumoto S. FGF23 beyond phosphotropic hormone. Trends Endocrinol Metab 2018;29:755-67. [Crossref] [PubMed]
- Erben RG. Update on FGF23 and klotho signaling. Mol Cell Endocrinol 2016;432:56-65. [Crossref] [PubMed]
- Malhotra R, Katz R, Hoofnagle A, et al. The effect of extended release niacin on markers of mineral metabolism in CKD. Clin J Am Soc Nephrol 2018;13:36-44. [Crossref] [PubMed]
- Kuro-O M. The Klotho proteins in health and disease. Nat Rev Nephrol 2019;15:27-44. [Crossref] [PubMed]
- Mencke R, Hillebrands JL. NIGRAM consortium. The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res Rev 2017;35:124-46. [Crossref] [PubMed]
- Lewin E, Olgaard K. The vascular secret of Klotho. Kidney Int 2015;87:1089-91. [Crossref] [PubMed]
- Yamada S, Giachelli CM. Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho. Bone 2017;100:87-93. [Crossref] [PubMed]
- Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone 2017;100:41-9. [Crossref] [PubMed]
- Rao M, Steffes M, Bostom A, Ix JH. Effect of niacin on FGF23 concentration in chronic kidney disease. Am J Nephrol 2014;39:484-90. [Crossref] [PubMed]
- Moher D, Schulz KF, Altman DG, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657-62. [Crossref] [PubMed]