The relationship between mean telomere length and blood pressure: results from the National Health and Nutrition Examination Surveys
Introduction
Hypertension has become a major public health problem in worldwide due to its close relationship with cardiovascular and cerebrovascular disease events (1,2). Blood pressure (BP) is a key indicator to reflect the function of cardiac output and systemic vascular resistance (3). The values of BP are normally distributed values in the general population (4). BP is considered to be a continuous, polygenic and heterogeneous trait (4), and an indicator for cardiovascular risk stratification because it is an easily measured and accessible parameter for routine data collection (5).
In recent years, emerging new biomarkers have been discovered and might play an important role in the development of hypertension. Telomere, a non-transcriptional region of repetitive nucleotide sequences at each end of a chromosome, protects chromosomes from degradation or from fusion with neighboring chromosomes (6). In addition, the length of telomere is a good gauge of cell aging and biological health, which significantly associates with longevity and the aging process (6,7). Mean telomere length (MTL) has significantly relationship with different age-related diseases, such as heart failure (8), diabetes mellitus (9), osteoporosis (10), atherosclerosis (11) and dementia (12). However, little research is available indicating if MTL is associated with the development of hypertension. The present study aimed to address the knowledge gap by examining the association of MTL with systolic/diastolic blood pressure (SBP/DBP) and the odds of hypertension in US adults.
Methods
Study design and study population
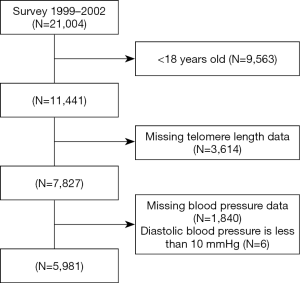
The National Health and Nutrition Examination Surveys (NHANES) is a cross-sectional and nationally representative survey of the civilian, non-institutionalized US population conducted by the Centers for Disease Control and Prevention (CDC). We used data from the 1999–2000 and 2001–2002 cycles of NHANES. Participants who aged ≥18 with test results of telomere length were included for analysis. After applying the exclusion criteria (Figure 1), 5,981 participants were available for analysis. The survey protocol was approved by the Institutional Review Board of the Centers for Disease Control and Prevention (Protocol #98-12). All participants have provided informed consent.
Measurement of BP
Three (or four for few participants) BP measurements (systolic and diastolic) were taken using a mercury sphygmomanometer in the mobile examination center (MEC) or during home examinations on all eligible individuals. For participants who were not able to complete examination in MEC (50 years and older, or less than 1 year of age) an abbreviated examination was conducted in their homes. BP measurements were taken by one of the MEC examiners. The procedures for BP measurement according to the latest recommendations of the American Heart Association Human Blood Pressure Determination by sphygmomanometers (13). Using diastolic reading as example, if all diastolic readings were zero, the average BP value would be zero. If there is one diastolic reading as zero and one (or more) non-zero values, the diastolic reading with zero was excluded from BP calculation. If two out of three diastolic readings are zero, the only non-zero diastolic reading was extracted for BP calculation. Two physicians (MEC setting) and two health technologists (Home Examination setting) were trained to collect NHANES BP data using a standardized protocol. Hypertension was defined as having a history of hypertension, a SBP ≥140 mmHg, a DPB ≥90 mmHg, and/or using antihypertensive medications (14).
Measurement of telomere length
Each sample was assayed 3 times on 3 separate days. The samples were assayed on duplicate wells, resulting in 6 data points. Sample plates were assayed in groups of 3 plates, and no 2 plates were grouped together for more than once. Each assay plate contained 96 control wells with 8 control deoxyribonucleic acid (DNA) samples. Assay runs with 8 or more invalid control wells were excluded from further analysis (<1% of runs). Control DNA values were used to normalize between-run variability. Runs with more than 4 control DNA values that deviated beyond 2.5 standard deviations from the mean for all assay runs were excluded from further analysis (<6% of runs). For each sample, any potential outliers were identified and excluded from the calculations (<2% of samples). The mean and standard deviation of the T/S ratio were then calculated. The inter assay coefficient of variation was 6.5%. Five 96-well quality control plates which represented 5% of the complete set were provided. These duplicate samples were blinded to the investigators. If more than 5% of the duplicate samples on the quality control plates were discordant with their pair in the complete set, the variant failed quality control (i.e., >95% duplicate concordance required). The MTL assay was performed in the laboratory at the University of California, San Francisco, using the quantitative polymerase chain reaction (PCR) method to measure telomere length relative to standard reference DNA (T/S ratio), as described in detail previously (15,16). Mean T/S ratio was measured by the telomere length relative to standard reference DNA. The formula to convert T/S ratio to base pairs was 3,274 + 2,413 × (T/S). The conversion from T/S ratio to bp was calculated based on a comparison of telomeric restriction fragment length from Southern blot analysis and T/S ratios using DNA samples from the human diploid fibroblast cell line IMR90 at different population doublings. The detailed information and methods are provided on the website (https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/TELO_A.htm).
Participants and clinical covariates
Demographics (including age, gender), previous medical history, smoking status, alcohol consumption and medication use (antihypertensive drugs, lipid lowering drugs and antidiuretic drugs) were collected from interviews. Anthropometric measurements, BP readings, blood and urine specimens, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), fasting blood glucose (FBG), C-reactive protein (CRP) and routine biochemical indicators were also obtained using standardized approach. Detailed description of data collection methods have been provided on the official website (https://www.cdc.gov/nchs/index.htm).
Statistical analysis
All continuous variables were presented as mean ± standard deviation, and categorical variables were presented in frequency or as a percentage according to baseline MTL in quartiles. The one-way ANOVA, Kruskal Wallis H test and Chi-square tests were used to determine any statistical differences between subgroups. Pearson correlation analysis was used to assess the relationship between MTL and clinical parameters. The association of MTL with continuous (SBP, DBP) and binary (the prevalence of hypertension) outcomes were examined using multivariate linear or logistic regression analysis. Results were presented in coefficients (betas), odds ratio (OR) with the corresponding 95% confidence intervals (CIs). To test the robustness of our results, we have built several regression models. In model I, we only included MTL. In model II we additionally adjusted for age, gender and body mass index (BMI). In model III, age, gender, BMI, FBG, SBP, TC, HDL-C, TG, LDL-C, CRP, estimated glomerular filtration rate (eGFR), alcohol consumption, smoking and antihypertensive drugs were adjusted. We also used generalized additive model (GAM) to identify the non-linear relationship between MTL and SBP, DBP and the odds of hypertension, then performed the likelihood ratio test. To address the nonlinearity of MLT and BP, a using multivariate linear or logistic regression analysis with cubic spline functions model and smooth curve fitting (penalized spline method) were conducted (17). If a nonlinearity was detected, we calculated the inflection point using a recursive algorithm, then a two-piecewise linear regression model was performed to calculate the threshold effect of the MTL on BP in terms of the smoothing plot (18). Recursive method was performed to calculate automatically the inflection point, where the maximum model likelihood would be used. A two-sided P<0.05 was considered statistically significant. All statistical analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline of participants
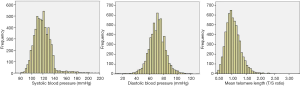
The characteristics of all the subjects in this study stratified by the quartiles of MTL were presented in Table 1. The distributions of BP and MTL were showed in Figure 2. A total of 5,981 participants [2,923 (48.9%) men] were included with average age as 45.2 years. There were 1,103 (18.4%) people with hypertension. In addition, there were significant subgroup differences in age, gender, BMI, SBP, DBP, FBG, TC, TG, LDL-C, HDL-C, CRP, eGFR, alcohol consumption, smoking status, hypertension and the use of antihypertensive drugs (all P<0.05).
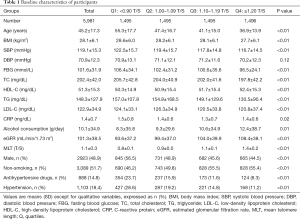
Full table
The relationship between telomere length and BP
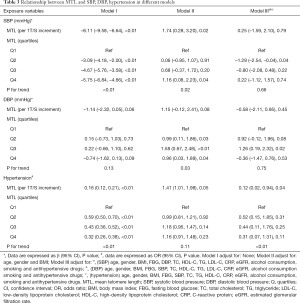
In Pearson correlation analysis (Table 2), MTL was significantly correlated with age, BMI, SBP, FBG, TC, TG, LDL-C, HDL-C, CRP, alcohol consumption and eGFR (all P<0.05). In order to further explore the association between MTL and SBP, DBP and the odds of hypertension, multivariate regression analysis were performed. As shown in Table 3, when treating MTL as a continuous variable (per 1 T/S increment), in the minimally adjusted model (model I), MTL was significantly associated with SBP (β=–8.11, 95% CI: –9.58, –6.64; P<0.01), DBP (β=–1.14, 95% CI: –2.32, 0.05; P=0.06) and the odds of hypertension (OR: 0.16, 95% CI: 0.12, 0.21; P<0.01). However, in the model III, there was no association between MTL and SBP (β=0.25, 95% CI: –1.59, 2.10, P=0.79), DBP (β=–0.58, 95% CI: –2.11, 0.95, P=0.45) but the association remained for the odds of hypertension (OR: 0.12, 95% CI: 0.02, 0.94; P=0.04). When using the lowest quartiles of MTL as the referent, multivariate linear (SBP, DBP)/logistic (hypertension) regression analyses demonstrated that the betas for SBP (βs were –1.29, –0.80, and 0.22 from the second to the fourth quartiles, respectively, P=0.66 for trend), DBP (βs were 0.92, 1.26, and –0.36 from the second to the fourth quartiles, respectively, P=0.75 for trend) and the odds of hypertension (ORs were 0.52, 0.44, and 0.31 from the second to the fourth quartiles, respectively, P<0.01 for trend) in model III.
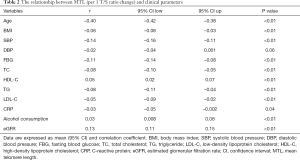
Full table
Full table
The analyses of non-linear relationship
Using a two-piecewise linear regression model, we calculated that the inflection point in T/S ratio for the association with SBP, DBP and hypertension to be 0.86, 1.02 and 0.80 respectively. On the left of the inflection point, the betas for SBP, DBP and the OR of hypertension prevalence were –12.58 (95% CI: –20.07, –5.09, P<0.01), 4.88 (95% CI: 1.29, 8.47, P<0.01) and 0.02 (95% CI: 0.001, 9.71, P=0.15) respectively. The corresponding effect estimates at the right of inflection point were 2.25 (95% CI: 0.07, 4.43, P=0.04), -3.30 (95% CI: –5.54, –1.06, P<0.01) and 0.26 (95% CI: 0.026, 2.60, P=0.25), respectively. Table 4, suggesting an inverse relationship between MTL and SBP on the left side of the inflection point, and an inverse relationship with DBP was on the right side with inflection point. As shown in Figure 3, the association between MTL and SBP (Figure 3A), DBP (Figure 3B) and the prevalence of hypertension (Figure 3C) were both non-linear after adjusting for potential confounding variables.

Full table

Discussion
In the present study, a non-linear relationship was found between MTL, SBP/DBP and the odds of hypertension. The optimal cutoff point of the association of MTL with SBP, DBP and hypertension were 0.86, 1.02 and 0.80 T/S respectively. On each side of the optimal MTL value, the magnitude of correlation between MTL and SBP, DBP and the prevalence of hypertension were different.
During recent years, a large number of studies have shown that MTL was significantly linked to cardiovascular risk factors, such as alcohol consumption (19), smoking (20), BMI (21) and blood lipids (22). Our results agreed with those previous studies, while demonstrating a saturation threshold for the relationship between MTL and elevated BP. Meanwhile, Aydos and his team members found no relationship between SBP, DBP, BMI, and telomere length by using of Spearman’s correlation coefficient (23). Demissie et al. (24) suggested that when comparing with normotensive peers, subjects with hypertension had a shorter age-adjusted telomere length in 327 men from the Framingham Heart study. However, the mechanism behind was still unclear. Our finding may provide a useful guide to future study.
It is possible that the longer the MTL, the lower risk for the hypertension development. The possible reasons can be as follows: on the one hand, telomere length was inversely related to aging, and which BP increases with age (25); on the other hand, telomere length was closely related to inflammation and oxidative stress, which played an important role in monitoring BP (26). In addition, shorter MTL may relate to the dysfunction of endothelial cells and vascular smooth muscle cells, insulin resistance hence the development of high BP (27).
However, some limitations should be taken into consideration for the present study. First, this study is cross-sectional in nature, therefore causality is not confirmed. Second, we did not examine markers related to inflammation and oxidative stress, which may also associate with hypertension (28,29). Third, the study population is limited to American population, the findings cannot be extrapolated to other populations. Despite these shortcomings, we have adjusted multiple confounding factors related to BP, and found a potential non-linear association between MTL and elevated BP. Finally, because this study only analyzed the relationship between BP and MLT, but not with telomere activity, the relationship between telomere activity and BP should be explored in the future.
In conclusion, a nonlinear association between MTL and elevated BP was found among US adults. Well-designed prospective studies are needed to clarify the causal relationship between them in the future.
Acknowledgments
We thank Chen Chao-Lei and Yu Yu-Ling to download the data.
Funding: This work was supported by the Science and Technology Plan Program of Guangdong Province (No. 2017B030314041), Science and Technology Plan Program of Guangzhou (No. 201604020143, No. 201604020018, No. 201604020186, and No. 201803040012), and the Key Area R&D Program of Guangdong Province (No. 2019B020227005).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.205). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The survey protocol was approved by the Institutional Review Board of the Centers for Disease Control and Prevention (Protocol #98-12). All participants gave written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217-23. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018;138:e426-83. [PubMed]
- Titze J, Luft FC. Speculations on salt and the genesis of arterial hypertension. Kidney Int 2017;91:1324-35. [Crossref] [PubMed]
- Seidel E, Scholl UI. Genetic mechanisms of human hypertension and their implications for blood pressure physiology. Physiol Genomics 2017;49:630-52. [Crossref] [PubMed]
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014;32:3-15. [Crossref] [PubMed]
- Whiteman VE, Goswami A, Salihu HM. Telomere length and fetal programming: A review of recent scientific advances. Am J Reprod Immunol 2017. [Crossref] [PubMed]
- Xu Y. Recent progress in human telomere RNA structure and function. Bioorg Med Chem Lett 2018;28:2577-84. [Crossref] [PubMed]
- Sharifi-Sanjani M, Oyster NM, Tichy ED, et al. Cardiomyocyte-specific telomere shortening is a distinct signature of heart failure in humans. J Am Heart Assoc 2017. [Crossref] [PubMed]
- Tamura Y, Takubo K, Aida J, et al. Telomere attrition and diabetes mellitus. Geriatr Gerontol Int 2016;16 Suppl 1:66-74. [Crossref] [PubMed]
- Valdes AM, Richards JB, Gardner JP, et al. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int 2007;18:1203-10. [Crossref] [PubMed]
- Benetos A, Aviv A. Ancestry, telomere length, and atherosclerosis risk. Circ Cardiovasc Genet 2017. [Crossref] [PubMed]
- Zhan Y, Song C, Karlsson R, et al. Telomere length shortening and Alzheimer disease--a mendelian randomization study. JAMA Neurol 2015;72:1202-3. [Crossref] [PubMed]
- Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation 1993;88:2460-70. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13-115. [PubMed]
- Needham BL, Adler N, Gregorich S, et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999-2002. Soc Sci Med 2013;85:1-8. [Crossref] [PubMed]
- Rippberger PL, Emeny RT, Mackenzie TA, et al. The association of sarcopenia, telomere length, and mortality: data from the NHANES 1999-2002. Eur J Clin Nutr 2018;72:255-63. [Crossref] [PubMed]
- Hu L, Hu G, Xu BP, et al. U-shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: a cohort study. J Clin Endocrinol Metab 2020. [Crossref] [PubMed]
- Yu X, Cao L, Yu X. Elevated cord serum manganese level is associated with a neonatal high ponderal index. Environ Res 2013;121:79-83. [Crossref] [PubMed]
- Wang H, Kim H, Baik I. Associations of alcohol consumption and alcohol flush reaction with leukocyte telomere length in Korean adults. Nutr Res Pract 2017;11:334-9. [Crossref] [PubMed]
- Astuti Y, Wardhana A, Watkins J, et al. Cigarette smoking and telomere length: a systematic review of 84 studies and meta-analysis. Environ Res 2017;158:480-9. [Crossref] [PubMed]
- An R, Yan H. Body weight status and telomere length in U.S. middle-aged and older adults. Obes Res Clin Pract 2017;11:51-62. [Crossref] [PubMed]
- Rehkopf DH, Needham BL, Lin J, et al. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross-sectional study of US adults. PLoS Med 2016;13:e1002188. [Crossref] [PubMed]
- Aydos SE, Tükün A. Does telomere length affect blood pressure? Adv Ther 2007;24:269-72. [Crossref] [PubMed]
- Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 2006;5:325-30. [Crossref] [PubMed]
- Rizvi S, Raza ST, Mahdi F. Telomere length variations in aging and age-related diseases. Curr Aging Sci 2014;7:161-7. [Crossref] [PubMed]
- Zhang J, Rane G, Dai X, et al. Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res Rev 2016;25:55-69. [Crossref] [PubMed]
- Fuster JJ, Díez J, Andrés V. Telomere dysfunction in hypertension. J Hypertens 2007;25:2185-92. [Crossref] [PubMed]
- Vasconcelos-Moreno MP, Fries GR, Gubert C, et al. Telomere length, oxidative stress, inflammation and BDNF levels in siblings of patients with bipolar disorder: implications for accelerated cellular aging. Int J Neuropsychopharmacol 2017;20:445-54. [Crossref] [PubMed]
- Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017;70:660-7. [Crossref] [PubMed]