
Fluorescence-guided surgery of the esophagus
Introduction
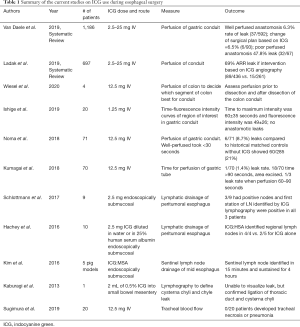
The use of indocyanine green (ICG) fluorescence near-infrared (NIR) imaging during gastrointestinal surgery has surged in recent years. Its use in foregut surgery, and specifically in esophageal surgery is the subject of active research. ICG is a tricarbocyanine iodide dye that can be safely injected intravenously or directly into tissue. When injected intravenously, it immediately binds plasma proteins, then is rapidly cleared by the liver and undergoes biliary excretion. These properties make it ideal for intraoperative angiography. Furthermore, its rapid biliary extraction allows repeated injections after relatively short washout periods. Its fluorescent properties are the result of molecular excitation brought about by use of near-infrared light at wavelengths exceeding 820 nm. It can be detected using specifically designed cameras during open or minimally invasive surgery (1). NIR imaging has several important applications in esophageal surgery including assessment of anastomotic perfusion, lymphatic mapping, and tracheal blood flow after mediastinal dissection. This is the first systemic review summarizing the current data on ICG use in esophageal surgery (Table 1).
Full table
ICG to assess perfusion of anastomosis
The most-studied application of ICG fluorescence imaging during esophageal surgery has been to evaluate the perfusion of the gastrointestinal-esophageal anastomosis. One of the most common and morbid complications of esophageal surgery is anastomotic leakage. There are several risk factors that can contribute to a higher risk of leak including nutritional status and prior radiation. Intraoperative factors such as tension, quality and length of the right gastroepiploic artery, and perfusion at the site of the anastomosis can also impact leak rate. The leak rate in esophageal surgery is reported to be between 5% and 20%. Recent data suggest 9% in intrathoracic anastomoses and 12% in cervical anastomoses (1,2). Traditionally, the visual appearance of the gastrointestinal tract, palpable pulse and bleeding from cut edges has been used to determine adequate perfusion. Adjunctive technology that provides objective perfusion assessment may potentially lower the risk of anastomotic leak. A recent meta-analysis shows a 10.9% leak rate when ICG is used (1). This is comparable to reported leak rates without the use of ICG angiography. However, when only studies that included a control group and a modification of surgical plan if ICG showed perfusion deficit, showed a 69% (89/436 vs. 15/261) absolute risk reduction of anastomotic leak.
Another recent systematic review showed that the anastomotic leak rate was 6.5% when there was a modification of the anastomosis based on ICG angiography findings. Whereas when there was ICG angiography evidence of poor perfusion but no intervention, there was 47.8% risk of anastomotic leak (2). Although promising, larger randomized prospective trials need to be completed to definitively establish the role of ICG angiography in preventing anastomotic leak.
While there are a number of reports regarding the use of ICG for evaluation of the esophago-gastric anastomosis, there is a dearth of evidence regarding the use of ICG for alternative reconstruction methods. A recent article described the use of ICG angiography in performing colonic interposition as esophageal reconstruction. The authors felt that ICG angiography played an important role in decision-making and can be used repeatedly during the procedure to make real-time decisions about which segment of colon is best-suited for reconstruction and to assess perfusion at the multiple sites of anastomosis (3).
Dose of ICG, consensus regarding the definition of “poor perfusion” as assessed by ICG and what intervention to perform to mitigate the risk of leak have all yet to be resolved.
Ishige et al. developed a technique of quantitatively measuring blood flow at planned anastomotic site. It involved using an IV dose of 1.25 mg then observing a small area of interest and measuring fluorescence intensity and time to reach maximum fluorescence. This was a small exploratory study and there were no anastomotic leaks (4). Noma et al. and Kumagai et al. published a 30- and 90-second rule respectively describing that well-perfused means it takes less than the allotted time to enhance with fluorescence (5,6). Noma et al. showed ICG angiography assessment of gastric conduit improved leak rate to 8.6% (6/71) compared to historical case-match control without ICG of 21% (60/285) using 12.5 mg ICG IV and a 30-second rule. It is unclear how many patients had interventions such as Kocherization, incision of hepatoduodenal ligament or revision of anastomosis. One patient had supercharge/drainage with cervical vessels of gastric conduit based on ICG angiography and that patient did not leak. Kumagai et al. showed 1.4% (1/70) leak rate using 12.5 mg ICG IV and 90-second rule. In 18/70 patients, gastric tube tip took >90-second to enhance with fluorescence so that portion was resected prior to anastomosis. In 3/70 patients, fluorescence of the gastric tube took 60–90-second and 1 of those patients had a minor leak. Both of these studies showed good results with low rates of anastomotic leak, however both studies were small and had low events.
Other applications of ICG in esophageal surgery
Given its simplicity and safety, alternate uses of ICG are actively being investigated during or following esophagectomy. Most of these reports are anecdotal or small case series, however as with any new technology, the more data that is being reported- the better the understanding of this new technology.
Lymphatic drainage assessment by ICG
Another application of ICG fluorescence imaging in esophageal surgery is for lymphatic mapping in esophageal cancer. In order to better characterize the lymphatic drainage and to guide dissection, ICG can be injected endoscopically submucosally near the tumor and NIR can be used to see first lymph node basins to enhance with fluorescence. Schlottmann et al. and Hachey et al. showed the feasibility of this technique in patients with gastroesophageal junction or mid-esophageal tumors (7,8). Schlottmann injected 2.5 mg submucosally in 4 quadrants of the esophagus near the tumor and lymph drainage was visualized 15–20 minutes after injection with NIR. The first lymph node station to fluoresce was the left gastric in 8/9 cases. Three out of nine cases had positive lymph nodes and first station lymph node identified by ICG lymphography were positive in all 3 patients. Hachey et al., used ICG:human serum albumin (ICG:HSA) in 4 patients and saw a trend towards better visualization of lymph nodes with NIR. More work needs to be done in this area to determine if a more limited lymph node dissection will have equivalent oncological outcomes. A limitation of ICG lymphography is that it doesn’t stay within lymph nodes for a long time and rapidly spreads to higher order lymph nodes. In order to improve fluorescence-guided sentinel lymph node biopsy, Kim et al., used a novel macrophage-targeting ICG bound to neomannosyl human serum albumin (ICG:MSA) in an animal model. The ICG:MSA compound is injected endoscopically into esophageal tissue. This targeted molecule did seem to improve sentinel lymph node identification in a porcine model (9).
ICG use in setting of post esophagectomy chylothorax
Kaburagi et al. report using ICG fluorescence imaging to guide treatment of chylothorax after esophagectomy. Two mL of 0.5% ICG was injected into small bowel mesentery to identify the thoracic duct and confirm transabdominal ligation (10).
ICG use for tracheal blood flow assessment
Sugimura et al. used ICG angiography to evaluate tracheal blood flow during esophagectomy. The authors hypothesized that extensive lymph node dissection and ligation of the bronchial artery may lead to tracheobronchial ischemia and increased risk of pulmonary complications. This small exploratory study showed feasibility, but was too low power to detect prediction of pulmonary complications (11).
Conclusions
ICG NIR imaging in esophageal surgery is becoming recognized as an important tool in the esophageal surgeons’ armamentarium. While early data supports its use in assessing the perfusion of the reconstructive anastomosis, larger randomized controlled trials are warranted to determine if ICG angiography can reduce the risk of anastomotic leak. Additional factors relating to the standardization and optimization of this technique also remain to be defined.
A number of novel applications of this technology including NIR lymphography, assessment of tracheal perfusion and identification of the thoracic duct, show promise. Overall, the use of NIR imaging in esophageal surgery may be a useful adjunct to current techniques, but it has not yet reached standard practice.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.138). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ladak F, Dang JT, Switzer N, et al. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 2019;33:384-94. [Crossref] [PubMed]
- Van Daele E, Van Nieuwenhove Y, Ceelen W, et al. Near-infrared fluorescence guided esophageal reconstructive surgery: A systematic review. World J Gastrointest Oncol 2019;11:250-63. [Crossref] [PubMed]
- Wiesel O, Shaw JP, Ramjist J, et al. The use of fluorescence imaging in colon interposition for esophageal replacement: A technical note. J Laparoendosc Adv Surg Tech A 2020;30:103-9. [Crossref] [PubMed]
- Ishige F, Nabeya Y, Hoshino I, et al. Quantitative Assessment of the blood perfusion of the gastric conduit by indocyanine green imaging. J Surg Res 2019;234:303-10. [Crossref] [PubMed]
- Noma K, Shirakawa Y, Kanaya N, et al. Visualized evaluation of blood flow to the gastric conduit and complications in esophageal reconstruction. J Am Coll Surg 2018;226:241-51. [Crossref] [PubMed]
- Kumagai Y, Hatano S, Sobajima J, et al. Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: Efficacy of the 90-second rule. Dis Esophagus 2018; [Crossref] [PubMed]
- Schlottmann F, Barbetta A, Mungo B, et al. Identification of the lymphatic drainage pattern of esophageal cancer with near-infrared fluorescent imaging. J Laparoendosc Adv Surg Tech A 2017;27:268-71. [Crossref] [PubMed]
- Hachey KJ, Gilmore DM, Armstrong KW, et al. Safety and feasibility of near-infrared image-guided lymphatic mapping of regional lymph nodes in esophageal cancer. J Thorac Cardiovasc Surg 2016;152:546-54. [Crossref] [PubMed]
- Kim HK, Quan YH, Oh Y, et al. Macrophage-targeted indocyanine green-neomannosyl human serum albumin for intraoperative sentinel lymph node mapping in porcine esophagus. Ann Thorac Surg 2016;102:1149-55. [Crossref] [PubMed]
- Kaburagi T, Takeuchi H, Oyama T, et al. Intraoperative fluorescence lymphography using indocyanine green in a patient with chylothorax after esophagectomy: report of a case. Surg Today 2013;43:206-10. [Crossref] [PubMed]
- Sugimura K, Miyata H, Shinno N, et al. Indocyanine green fluorescence imaging of the tracheal blood flow during esophagectomy. J Surg Res 2019;241:1-7. [Crossref] [PubMed]


