
Looking for solutions to lung dysfunction in type 2 diabetes
Diabetes-induced pulmonary dysfunction is an emerging topic within the “new” complications of diabetes and in particular in type 2 diabetes (1). The lungs show a huge vascularization and are rich in proteins with an elevated turnover such as elastin and collagen, pointing this organ as a potential target to the harmful effects of chronic hyperglycaemia (1). In fact, an inverse association between metabolic control and spirometric values has been observed (2). In addition, positive changes in spirometric maneuvers after of 3-months of improvement in glycemic control has also been reported (3). The mechanisms to explain the cluster of pulmonary dysfunctions associated with type 2 diabetes are not yet fully understood. However, it has been argued that the most suitable explanations could be related with insulin resistance, nonenzymatic glycosylation of lung proteins, low-grade chronic inflammation state, microvascular damage, autonomic neuropathy and defects in the bronchiolar surfactant layer (1) (Figure 1). The manuscript of Kim et al. sheds light on this issue, exploring the connection between prediabetes, type 2 diabetes and pulmonary function in the Korea National Health and Nutrition Examination Survey (KNHANES) (4). Their data confirm not only that patients with type 2 diabetes exhibits lower values of forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) than control subjects, but also that the impaired pulmonary function also exists in the prediabetes stage. Overall, data from Kim et al. confirm previous information in Caucasian population and reinforce the notion that lung dysfunction is a progressive defect across glucose abnormalities, beginning in the prediabetes stage and expanding when type 2 diabetes appears (5). Notably, after adjustment for potential confounding factors (age, sex, body mass index, waist circumference and smoking status) an increase of 1% in the HbA1c level was associated with a −1.20% difference in FVC and a −0.77% difference in FEV1, with similar results when a 10 mg/dL increase in fasting plasma glucose was considered. As prevalence of prediabetes and undiagnosed diabetes are both increasing, a closer relationship between endocrinologists, pneumologists and primary care physicians is needed not only to better understand the deleterious effects of type 2 diabetes on lung parenchyma but to successfully target pulmonary dysfunction (6).
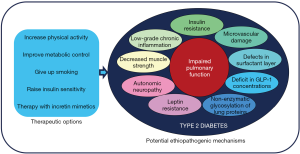
The potential advantageous role of physical activity on the respiratory muscles’ strength and function has been reported in patients with lung diseases (7). Our group has also assessed the benefit from heart-healthy lifestyle behaviors on lung mechanics in a cross-sectional study conducted in 3,020 Spanish middle-aged subjects free of lung disease (8). In this population, low physical activity was significant and independently associated with the presence of pulmonary impairment assessed by FEV1 <80% only in men (8). Kim et al. go one step further to explore the therapeutic implications of walking exercise in preventing a decreased pulmonary function in subjects with diabetes (4). Their results show that walking more than 300 minutes per week has a significant effect avoiding FVC and FEV1 decline. However, this beneficial effect disappeared after correction for smoking status, pointing to smoking cessation as a fundamental pillar among the strategies aimed at improving lung function of patients with diabetes.
Other possibilities that can be added to physical activity to treat or prevent lung dysfunction in patients with type 2 diabetes should be considered. The first option to consider would be the improvement of glycemic control. In this way, Gutiérrez-Carrasquilla et al. have recently communicated results from a prospective and interventional study to determine whether ameliorating metabolic control in patients with type 2 diabetes without known pulmonary disease during a three-month period produce significant changes in respiratory function (3). Therefore, in the Sweet Breath Study a favorable change in the spirometric parameters was just observed in the subgroup of participants who reached a reduction of their HbA1c higher than 0.5%, and this result was not related with weight reduction (3). More interesting, the spirometric parameters that appeared to be most sensitive to this rapid improvement of metabolic control were peak expiratory flow (PEF) and FEV1, the former related with neuromuscular integrity. This relation between muscle strength and pulmonary function also reinforce the relevance to prescribe physical activity to patients with type to diabetes more vulnerable to develop lung involvement. And it is also another reason to insist on patients with diabetes about the need to achieve good glycemic control and thus prevent the development of late complications.
The glucagon like peptide 1 (GLP-1) receptor is expressed by alveolar type 2 cells, and its activation have been shown to stimulate the production of pulmonary surfactant in experimental studies (9). In fact, serum surfactant protein D (SP-D) has been proposed as a serum biomarker useful to identify patients with type 2 diabetes with defects in their bronchiolar surfactant layer (10). In this way, SP-D serum concentrations were inversely correlated with FEV1 and the stepwise multivariate regression analysis showed that a serum SP-D value equal or higher than 32.3 ng/mL was independently associated with a FEV1 <80% of predicted (10). Therefore, the underlying deficit of GLP-1 in type 2 diabetes could also be involved in the impairment of airway caliber. This hypothesis is now been testing in the LIRALUNG study (ClinicalTrials.gov Identifier: NCT02889510), a randomized double blind, crossover, placebo controlled clinical trial to evaluate the effect of liraglutide, a GLP-1 analogue, on lung function in patients with type 2 diabetes. On the other hand, worthy of additional attention is whether preventing the inactivation of the endogenous GLP-1 through the pharmacological inhibition of the dipeptidyl peptidase-4 applies any effect on pulmonary function (1). On a similar level remains the possibility to enhance pulmonary function rising insulin sensitivity in patients with type 2 diabetes. Although there are no prospective studies addressing this option, a little retrospective study also in Korean patients showed how treatment with insulin sensitizers was independently associated with improvements in FVC compared with insulin therapy (11). And similarly, after adjustment for glycemic control and the known duration of type 2 diabetes, Colombian patients under treatment with metformine showed significantly lower differences from the expected values in FVC measures in comparison with patients receiving a secretagogue therapy (12).
To sum up, we hope that health professionals who take care of patients with type 2 diabetes begin to consider them as a vulnerable group for pulmonary dysfunction. Although clinical relevance of such changes has to date been little, we cannot forget that a 10% decrease in FEV1 is an independent predictor of all-cause mortality in type 2 diabetes (13). In this context, and while we find therapeutic targets capable of reversing this situation, investment in a healthy lifestyle that includes high physical activity, give up smoking and selected antidiabetic therapies to improve metabolic control should be recommended to patients with diabetes.
Acknowledgments
Funding: This research was supported by grants from the Instituto de Salud Carlos III (Fondo de Investigación sanitaria, PI 15/00260 and PI 18/00964) and the European Union (European Regional Development Fund).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.225). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lecube A, Simó R, Pallayova M, et al. Pulmonary Function and Sleep Breathing: Two New Targets for Type 2 Diabetes Care. Endocr Rev 2017;38:550-73. [Crossref] [PubMed]
- Lecube A, Sampol G, Muñoz X, et al. Type 2 diabetes impairs pulmonary function in morbidly obese women: a case-control study. Diabetologia 2010;53:1210-6. [Crossref] [PubMed]
- Gutiérrez-Carrasquilla L, Sánchez E, Barbé F, et al. Effect of Glucose Improvement on Spirometric Maneuvers in Patients With Type 2 Diabetes: The Sweet Breath Study. Diabetes Care 2019;42:617-24. [Crossref] [PubMed]
- Kim JM, Kim MK, Joung KH, et al. Association between glycemic state and pulmonary function and effect of walking as a protective factor in subjects with diabetes mellitus. Ann Transl Med 2019;7:530. [Crossref] [PubMed]
- Sánchez E, Gutiérrez-Carrasquilla L, Barbé F, et al. Lung function measurements in the prediabetes stage: data from the ILERVAS Project. Acta Diabetol 2019;56:1005-12. [Crossref] [PubMed]
- Mirahmadizadeh A, Fathalipour M, Mokhtari AM, et al. The prevalence of undiagnosed type 2 diabetes and prediabetes in Eastern Mediterranean region (EMRO): A systematic review and meta-analysis. Diabetes Res Clin Pract 2020;160:107931. [Crossref] [PubMed]
- Salcedo PA, Lindheimer JB, Klein-Adams JC, et al. Effects of Exercise Training on Pulmonary Function in Adults With Chronic Lung Disease: A Meta-Analysis of Randomized Controlled Trials. Arch Phys Med Rehabil 2018;99:2561-9.e7. [Crossref] [PubMed]
- Gutiérrez-Carrasquilla L, Sánchez E, Hernández M, et al. Effects of Mediterranean Diet and Physical Activity on Pulmonary Function: A Cross-Sectional Analysis in the ILERVAS Project. Nutrients 2019. [Crossref] [PubMed]
- Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, et al. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology 2015;156:3559-69. [Crossref] [PubMed]
- López-Cano C, Lecube A, García-Ramírez M, et al. Serum surfactant protein D as a biomarker for measuring lung involvement in obese patients with type 2 diabetes. J Clin Endocrinol Metab 2017;102:4109-16. [Crossref] [PubMed]
- Kim HJ, Lee JY, Jung HS, et al. The impact of insulin sensitisers on lung function in patients with chronic obstructive pulmonary disease and diabetes. Int J Tuberc Lung Dis 2010;14:362-7. [PubMed]
- Vargas HA, Rondón M, Dennis R. Pharmacological treatment and impairment of pulmonary function in patients with type 2 diabetes: a cross-sectional study. Biomedica 2016;36:276-84. [Crossref] [PubMed]
- Davis WA, Knuiman M, Kendall P, et al. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 2004;27:752-7. [Crossref] [PubMed]


