
Decreased Foxp3 and function of Tregs caused immune imbalance and liver injury in patients with autoimmune liver diseases post-liver transplantation
Introduction
Currently, liver transplantation is the most effective treatment for acute and chronic liver failure when medical options are no longer available (1,2). Autoimmune liver diseases (AILD) is a type of autoimmune disease mainly composed of autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) which may cause end-stage liver failure and require liver transplantation. In the USA, 24% of liver transplant patients are patients with AILD (3).
Regulatory T cells (Tregs) play an irreplaceable role in maintaining immunological homeostasis. Two main subsets of Tregs have been well described: thymus-derived natural Tregs (nTregs) and induced Tregs (iTregs). Interleukin (IL)-2 and transforming growth factor (TGF)-β can induce the formation of iTregs from CD4+ naïve T cells in vitro (4). Tregs play a key role in maintaining the balance and tolerance in transplant patients and sustaining the graft function in patients. Tregs have been proven to be important in regulating alloreactive immunity after solid organ transplantation in several studies (5,6). For example, Treg frequency is related to the long-term graft survival one year after kidney transplantation (7). Tregs tend to decline after transplantation, but their suppressive capacity is maintained or even enhanced against donor antigens (8). Studies in kidney transplant recipients indicated increased FoxP3 expression negatively correlated with acute rejection incidence (9), Louis observed reduced Treg frequencies in peripheral blood during chronic rejection (10). Similarly, Tregs after bone marrow transplantation had been related to acute and chronic graft-versus-host disease (11,12), with reduced Treg frequencies possibly being involved in the development of chronic graft-versus-host disease (13-15). However, Specific post-transplant issues in patients transplanted for AILD are the recurrence of the original disease and a higher rate of rejection, which may both impact on survival (16).
Here, we demonstrate that low Foxp3 expression and unstable functionality may be the cause of the immune imbalance and decreasing of graft function. Improving the function of Tregs may be a novel therapeutic strategy to prolong the graft function and benefit to those post-transplantation patients with AILD.
Methods
Patients
Sixty-nine patients with liver transplantation during between June 2012 and February 2019 who had had a clear clinical diagnosis of failure, including hepatitis B virus (HBV)-induced liver cirrhosis or AILD, were used as research subjects. Indications of LT were the complications of end-stage liver diseases, including hepatic encephalopathy, upper gastrointestinal hemorrhage, spontaneous bacterial peritonitis, hepatorenal syndrome and refractory ascites. The local ethics committee approved the study. Informed consent was obtained from all participants.
Specimen collection
When the patients were admitted to the hospital, we collected fasting blood at 7:00 in the morning without any treatments. These samples would be used for biochemical indicators and immune cell phenotype determination.
For the immune cell phenotype determination, peripheral blood mononuclear cells (PBMCs) were surface-stained with CD8 to evaluate the proportion of CD8+T cells. For Tregs analysis, PBMCs were stained with CD4, CD25, and CD127, while CD4+CD25+CD127- were considered Tregs.
For the function analysis of Treg, PBMCs were prepared from heparinized venous blood of transplant patients from both groups by Ficoll-Hypaque density gradient centrifugation. Human nTregs were sorted from PBMCs by gating on CD4+CD25brightCD127- cells (>97% purity). nTregs were activated and expanded with anti-CD3/CD28 beads (1 bead to 3 cells) and IL-2 (300 U/mL) for 10 days.
Observation indicators
Biochemical indicators for liver function such as ALT and AST, was detected through Beckman Coulter AU5800 automatic biochemical analysis system. The phenotype of the immune cells for the patient was analyzed through FACS.
Suppressive assays in vitro
Peripheral blood mononuclear cells (PBMCs) were prepared from heparinized venous blood of healthy adult volunteers by Ficoll-Hypaque density gradient centrifugation. PBMC were labeled with CFSE (InVitrogen). Anti-CD3 mAb-coated beads (Dynal) were added in a 1:1 ratio (bead: PBMC), and nTregs were added at different ratios. Finally, cultures were incubated at 37 °C. On day 4, cells were stained with anti-CD8 APC. Data were acquired and analyzed using the proliferation platform in FlowJo, and the suppression index was determined using Division Index (17).
Flow cytometry
For extracellular staining, harvested cells were washed and incubated in PBS containing 1% FBS containing the below fluorochrome-conjugated antibodies in a flow tube. For intracellular staining of cytokines, cells were stimulated with phorbol 12-myristate 13-acetate (50 ng/mL, Biogems), ionomycin calcium salt (1 µg/mL, Biogems), and brefeldin A (5 µg/mL, Biogems) for 6 h. Then, cells were stained with surface markers and further fixed/permeabilized (BioLegend) and stained for intracellular protein. Human-specific monoclonal antibodies used for flow cytometry included CD4 (A161A1), CD25 (BC96), Foxp3 (206D), CD45RA (HI100), purchased from BioLegend, and CD127 (A019D5) purchased from BD Pharmingen. Sample detection was performed by MACSQuant Analyzer 10 (Miltenyi Biotec, Germany) and data were analyzed with FlowJo software.
Xeno-GVHD model generation
NOD/SCID/IL2r common γ chain-/-(NOG) mice were obtained from Jackson Laboratory. The mice were bred and housed under specific pathogen-free conditions in microisolator cages and given unrestricted access to autoclaved food and sterile water. Animals of both sexes were used for experiments at 8–12 weeks of age. The mice received a single dose of 200 cGy gamma irradiation from a linear accelerator before the injection of human PBMC on the same day (18). All experiments were performed according to the guidelines of the Institutional Animal Committee of Nanjing Medical University.
Western blot analysis
Proteins were extracted from harvested cells, and their concentration was determined by the BCA assay (pierce). Protein samples (30 µg) were resolved by SDS-PAGE and transferred to a PVDF membrane. The following antibodies were used: STAT1 (Cell signaling technology, #9170), STAT3 (Santa cruz biotechnology, SC-8019), p-STAT1 (Cell signaling technology, #7649), and p-STAT3 (Santa cruz biotechnology, SC-81523). The results were visualized with Kodak autoradiography film (Kodak XAR film).
TSDR methylation status assay
Genomic DNA was isolated using the Mammalian Genomic DNA Extraction kit (Beyotime) and processed by using the EZ DNA Methylation-Direct kit (Zymo Research) according to the manufacturer’s protocol. Purified bisulfite-treated DNA was used in bisulfite sequencing PCR with the following a pair of TSDR amplification PCR primers: 5'-TTG GGT TAA GTT TGT TGT AGG ATA G-3' and 5'-ATC TAA ACC CTA TTA TCA CAA CCC C-3'. The PCR products were purified and cloned into pMD-18T vector (Takara) and single clones were selected for sequencing. All sequencing results of bisulfite- converted TSDR region were analyzed on BDPC DNA methylation analysis platform, and the average methylation status of 11 CpG sites in TSDR region was statistically analyzed.
Statistical analysis
All research data were imported into excel form, statistical analysis was performed using SPSS software, and charts were made with Graphpad 6.0. The counting data obtained from the study were uniformly processed by paired t-test analysis, linear regression analysis and nonlinear regression analysis. Differences in Kaplan-Meier survival curves were analyzed by the log-rank test. Analyze the data with a statistical difference of P<0.05 or P<0.01.
Results
Patient information
A total of 69 patients who underwent liver transplantation in The Liver Transplantation Center of the First Affiliated Hospital of Nanjing Medical University from June 2012 to February 2019 were included in the study, which comprised 41 males and 28 females. The mean age of the patients was 52.7 years; 4.3% of the patients were younger than 30 years old, while 20.3% were older than 60 years. Among the patients, 47 patients have had a hepatic viral infection (68.1%) while 22 had autoimmune (31.9%) disease, which caused liver failure before transplantation. Furthermore, 17.4% of the patients presented abnormal hepatic function (ALT or TBIL >1.5 folds of the local laboratory upper limit of normal) (Table 1).
Full table
Liver function and immune cell phenotype were modulated for patients after liver transplantation
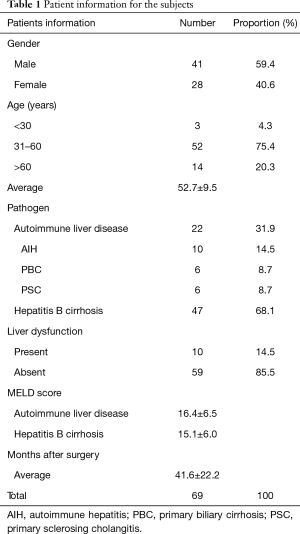
The serum ALT and TBIL levels were considered as important markers of liver graft function post-liver transplantation (19,20) and correlated with the extent of hepatocellular necrosis (21) and biliary tract functionality (22,23). Thus, we analyzed both the ALT and TBIL of the patients’ post-transplantation from the HBV group or the AILD group. Our data showed that AILD presented higher levels of ALT, AST and TBIL compared with the HBV group (ALT: 90.8±61.9 to 38.5±31.7, TBIL: 76.3±143.5 to 9.9±5.1, P value <0.001) (Figure 1A). Moreover, we also investigated the percentage of the patients with abnormal ALT or TBIL in a different group, which indicated that there was both a higher ALT and TBIL level in the AILD group compared to the HBV group (ALT: 27.3% to 8.5%, TBIL: 18.2% to 4.4%, P value <0.001) (Figure 1B). We also analyzed both the ALB, INR and Cr of the patients post-transplantation from the HBV group and the AILD group. Our data showed that AILD presented higher levels of INR compared with the HBV group, lower levels of ALB compared with the HBV group and the levels of Cr had no statistical difference in two groups (Figure S1).
We also tested immune cell phenotypes like CD8 and Tregs for different groups. The unique structure of the hepatic tissue allows direct activation of alloreactive naïve CD8 T cells within the liver graft itself, long-term donor chimerism, Tregs and soluble allo-MHC molecules secreted by the liver allograft (24). Tregs, mainly CD4+CD25+FoxP3+ T cells, are responsible for the maintenance of allograft tolerance following transplantation of various organs (25). No significant difference was observed in CD8 T cells for different groups (Figure 1C); surprisingly, we observed higher Tregs in the AILD group compared with the HBV group (Figure 1D). CD8/Treg can also be an essential marker for immune balance and tolerance (26,27). Thus we calculated the level of CD8/Treg from different groups, which proved that CD8/Treg was significantly decreased in the AILD group (2.3±1.1) compared with the HBV group (4.4±1.9) (Figure 1E).
Treg and CD8/Treg fully corrected to the graft function in AILD patients post-transplantation
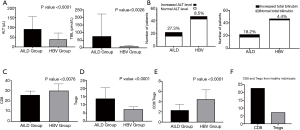
We verified CD8, Treg, and CD8/Treg to the graft function, including ALT and TBIL. While no correlation was observed between CD8 and TBIL, CD8 showed a negative correlation to ALT (Figure 2A), Treg showed a strong positive correlation to ALT or TBIL in the AILD group post-transplantation (Figure 2B). Furthermore, a negative correlation was also detected between CD8/Treg to ALT or TBIL (Figure 2C). We also analyzed the relative correlation level in the HBV group; no significant correlation was detected (Figure S2A,B,C).


AILD-Treg expresses less Foxp3 and is not stable during culturing compared to HBV-Treg
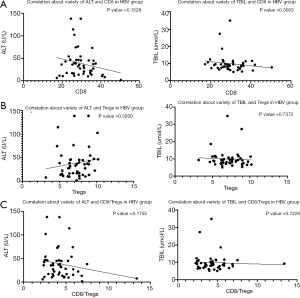
Previous data surprisingly determined the percentage of CD4+CD25+CD127− subset was negatively correlated with graft function in ALID patients post-transplantation. It was previously thought that Tregs play an essential role in maintaining immune balance and graft function during organ transplantation (25). Thus, we isolated nTregs from AILD and HBV groups through sorting CD4+CD25brightCD127- cells (>97% purity) from PBMCs of the patients. As Foxp3 is the key marker for Tregs (28), we evaluated the expression of Foxp3 from both groups. Our data showed that Tregs from the AILD group (AILD-Tregs) presented lower Foxp3 expression compared with Tregs from the HBV group (HBV-Tregs) (Figure 3A,B). We also expanded the Tregs for further experience and analyzed the stability of Foxp3 expression for both Treg subsets. Tregs were cultured with the addition of IL-2, anti-CD3/CD28 beads for 10 days, and IL-2 and culture medium were added every three days to maintain the concentration of cells during culture. The results were that both Tregs loss Foxp3 expression while AILD-Tregs decreased more Foxp3 compared with HBV-Tregs (Figure 3C). The CD45RA staining showed that the percentage of CD45RA+ T cells in two groups had no statistical difference (Figure 3D).
Tregs presented lower suppressive ability both in vitro and vivo in AILD group compared with HBV group post-transplantation
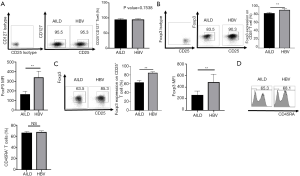
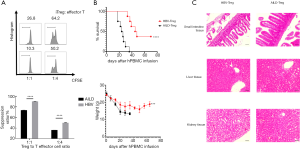
PBMC isolated nTreg from AILD or HBV group were cultured for 10 days, and then, cells were harvested. CFSE co-culture assays were performed to estimate the suppressive ability of the two Treg subsets. nTreg were co-incubated with CFSE-labeled fresh PBMC in the presence of anti-CD3 beads. HBV-Tregs showed stronger suppressive activity in any ratio contrasting to AILD-Tregs (Figure 4A). We next analyzed the immunosuppressive ability of nTreg from both groups in vivo in a mouse xenogeneic GVHD model. Co-transfer of HBV-Tregs with PMBCs significantly prolonged mouse survival and delayed severe weight loss compared to the AILD-Treg group (Figure 4B), and pathological examination proved there was more critical tissue damage and infiltration in the small intestine, liver, and kidney in the AILD-Treg groups compared with the HBV-Treg group (Figure 4C).
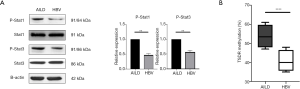
Higher STAT1 and STAT3 phosphorylation and CpG methylation was detected in AILD-nTreg compared with HBV-nTreg
To further examine the underline mechanism by which AILD-Tregs express lower Foxp3 and are unstable, and not as functional as HBV-Tregs in vitro and vivo, we investigated the expression and activation of the downstream signaling during inflammatory cytokine stimulation. It has been well recognized that IL-1 and/or IL-6 exert their effects through the activation of STAT1 and STAT3, respectively (29-31). We observed that AILD-Tregs markedly improved STAT1 and STAT3 activation compared to HBV-Tregs (Figure 5A). The epigenetic regulation in the Foxp3 locus leading to demethylation of CpG islands in the region of Foxp3 locus is considered to be an essential hallmark for the stability and functionality of Foxp3+ Tregs (32). We used bisulfite sequence analysis to examine the methylation status of both nTreg. As expected, a higher methylation level was detected in AILD-nTreg (Figure 5B).
Discussion
AILD is a type of autoimmune disease that may cause liver injury or failure. Evidence for the loss of central immune tolerance in AIH comes from murine studies (33-35), and inducing non-specific T-cell activation in mice has been shown to result in T-cell-mediated liver injury (36). In the development of PBC, specific loss of immune tolerance to a mitochondrial antigen, the lipoyl domain of the immunodominant E2 component of pyruvate dehydrogenase (PDC-E2) subunit is archetypal (37). In PSC, there is a clear T-cell predominant infiltrate (38) but little knowledge to date of relevant auto-antigenic triggers. There is no curative treatment for all three disorders, and a considerable number of patients eventually progress to an end-stage liver disease requiring liver transplantation (LT). LT, in this context, has a favorable overall outcome with the current patient and graft survival exceeding 80% at 5 years (16).
In this study, we made an analysis comparing the immune balance and graft function between AILD patients’ post-transplantation and the patients who have had a liver failure with HBV infection post-transplantation. Our data showed that there is a lower prognosis with severe graft function in liver transplant patients with AILD compared to those patients with HBV-induced liver failure. Immune cell phenotype analysis showed that more Tregs could be detected in AILD patients compared with HBV patients’ post-transplantation. We sorted CD4+CD25+CD127- Tregs in vivo and showed that Tregs presented decreased function both in vitro and vivo. Mechanism study also proved that modulation of the phosphorylation level of STAT1 and STAT3 in addition to the methylation level of TSDR in Foxp3 might have partially resulted in the expression and function loss of Tregs. These results suggest that function loss of Tregs may be the critical factor that causes graft loss for liver transplant patients after AILD.
Our clinic study data verified that CD8/Treg positive correlated to the graft function post-transplantation while Treg negatively correlated to the graft function. This data is conflicted with the previous result indicating that Tregs protect and reduce liver failure in AILD (39). As the low percentage of Foxp3+ Tregs in the blood, we usually evaluated the CD4+CD25+CD127- Tregs to evaluate the Foxp3 expression in the clinic. However, CD4+CD25+CD127− Tregs may not be suitable for presenting the percentage of Foxp3+ cells in AILD patients post-transplantation due to CD4+CD25+CD127− Tregs lost Foxp3 expression in vivo; in addition to further analyzing the Foxp3 expression and suppressive function in vivo and vitro, this is why we sorted the Tregs from AILD patients. The experimental data proved that Tregs were not functional in AILD patients due to low expression of Foxp3, while our published data indicated that low Foxp3+ Tregs might be able to induce instead of suppressing inflammatory reactions. Meanwhile, increasing low functional Tregs may aggravate the inflammatory disorder, which finally leads to graft function loss. Besides, we found that CD8+ T cells is positive correlated to the graft function post-transplantation, the result is surprising, but we still need more investigation on the function of the improved CD8+ T cells.
Previously, several studies had already estimated mechanisms that may be related to Treg function and stability in vitro and vivo. Liu found that increased phosphorylation of STAT3 resulted in Foxp3 and IL-10 expression in Tregs (40). Keohane reported JAK induces silencing of T Helper cytokine secretion and a profound reduction in T regulatory cells (41). Harusato demonstrated NFκB activation and STAT transcription might also regulate the induction of Tregs (42).
We proved that AILD-Tregs expressed higher STAT1 and STAT3 phosphorylation and CpG methylation compared with HBV-nTreg. This may partly explain why lower Foxp3 expression and Treg function was observed in AILD-Tregs but not HBV-Tregs.
Our findings in this study present a new understanding of Tregs and immune balance in AILD patients’ post-transplantation. Further characterization of the mechanisms underlying the conversion and function of plastic Foxp3+ T cells in AILD patents is needed for the development of new therapeutic strategies to preserve graft function and reduce liver injury for patients with autoimmune diseases post-liver transplantation.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (No. 81530048 and No. 31700791).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.203). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Animal Committee of Nanjing Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seshadri RM, Besur S, Niemeyer DJ, et al. Survival analysis of patients with stage I and II hepatocellular carcinoma after a liver transplantation or liver resection. HPB (Oxford) 2014;16:1102-9. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Ilyas JA, O'Mahony CA, Vierling JM. Liver transplantation in autoimmune liver diseases. Best Pract Res Clin Gastroenterol 2011;25:765-82. [Crossref] [PubMed]
- Zheng SG, Wang J, Wang P, et al. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007;178:2018-27. [Crossref] [PubMed]
- Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol 2010;6:577-83. [Crossref] [PubMed]
- Boros P, Bromberg JS. Human FOXP3+ regulatory T cells in transplantation. Am J Transplant 2009;9:1719-24. [Crossref] [PubMed]
- San Segundo D, Fernandez-Fresnedo G, Rodrigo E, et al. High regulatory T-cell levels at 1 year posttransplantation predict long-term graft survival among kidney transplant recipients. Transplant Proc 2012;44:2538-41. [Crossref] [PubMed]
- Sewgobind VD, van der Laan LJ, Klepper M, et al. Functional analysis of CD4+ CD25bright T cells in kidney transplant patients: improving suppression of donor-directed responses after transplantation. Clin Transplant 2008;22:579-86. [Crossref] [PubMed]
- Veronese F, Rotman S, Smith RN, et al. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant 2007;7:914-22. [Crossref] [PubMed]
- Louis S, Braudeau C, Giral M, et al. Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation 2006;81:398-407. [Crossref] [PubMed]
- Ukena SN, Velaga S, Geffers R, et al. Human regulatory T cells in allogeneic stem cell transplantation. Blood 2011;118:e82-92. [Crossref] [PubMed]
- Dong S, Maiella S, Xhaard A, et al. Multiparameter single-cell profiling of human CD4+FOXP3+ regulatory T-cell populations in homeostatic conditions and during graft-versus-host disease. Blood 2013;122:1802-12. [Crossref] [PubMed]
- Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005;106:2903-11. [Crossref] [PubMed]
- Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood 2006;108:1291-7. [Crossref] [PubMed]
- Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood 2004;104:2187-93. [Crossref] [PubMed]
- Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol 2014;60:210-23. [Crossref] [PubMed]
- Lu Y, Wang J, Gu J, et al. Rapamycin regulates iTreg function through CD39 and Runx1 pathways. J Immunol Res 2014;2014:989434.
- Covassin L, Laning J, Abdi R, et al. Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rgamma(null) H2-Ab1 (tm1Gru) Tg (human leucocyte antigen D-related 4) mice: a mouse model of human allogeneic graft-versus-host disease. Clin Exp Immunol 2011;166:269-80. [Crossref] [PubMed]
- Strasberg SM, Howard TK, Molmenti EP, et al. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology 1994;20:829-38. [Crossref] [PubMed]
- Azoulay D, Del Gaudio M, Andreani P, et al. Effects of 10 minutes of ischemic preconditioning of the cadaveric liver on the graft's preservation and function: the ying and the yang. Ann Surg 2005;242:133-9. [Crossref] [PubMed]
- Field KM, Dow C, Michael M, Part I. Liver function in oncology: biochemistry and beyond. Lancet Oncol 2008;9:1092-101. [Crossref] [PubMed]
- Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J 2003;79:307-12. [Crossref] [PubMed]
- Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol 2000;32:508-15. [Crossref] [PubMed]
- Benseler V, McCaughan GW, Schlitt HJ, et al. The liver: a special case in transplantation tolerance. Semin Liver Dis 2007;27:194-213. [Crossref] [PubMed]
- Waldmann H, Hilbrands R, Howie D, et al. Harnessing FOXP3+ regulatory T cells for transplantation tolerance. J Clin Invest 2014;124:1439-45. [Crossref] [PubMed]
- Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538-43. [Crossref] [PubMed]
- Azzimonti B, Zavattaro E, Provasi M, et al. Intense Foxp3+ CD25+ regulatory T-cell infiltration is associated with high-grade cutaneous squamous cell carcinoma and counterbalanced by CD8+/Foxp3+ CD25+ ratio. Br J Dermatol 2015;172:64-73. [Crossref] [PubMed]
- Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 2011;11:119-30. [Crossref] [PubMed]
- Joshi VD, Kalvakolanu DV, Chen W, et al. A role for Stat1 in the regulation of lipopolysaccharide-induced interleukin-1beta expression. J Interferon Cytokine Res 2006;26:739-47. [Crossref] [PubMed]
- Sawa S, Kamimura D, Jin GH, et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med 2006;203:1459-70. [Crossref] [PubMed]
- Nguyen H, Chatterjee-Kishore M, Jiang Z, et al. IRAK-dependent phosphorylation of Stat1 on serine 727 in response to interleukin-1 and effects on gene expression. J Interferon Cytokine Res 2003;23:183-92. [Crossref] [PubMed]
- Floess S, Freyer J, Siewert C, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007;5:e38. [Crossref] [PubMed]
- Scheiffarth F, Warnatz H, Mayer K. Studies concerning the importance of mononuclear cells in the development of experimental hepatitis. J Immunol 1967;98:396-401. [PubMed]
- Mori Y, Mori T, Yoshida H, et al. Study of cellular immunity in experimental autoimmune hepatitis in mice. Clin Exp Immunol 1984;57:85-92. [PubMed]
- Kuriki J, Murakami H, Kakumu S, et al. Experimental autoimmune hepatitis in mice after immunization with syngeneic liver proteins together with the polysaccharide of Klebsiella pneumoniae. Gastroenterology 1983;84:596-603. [Crossref] [PubMed]
- Tiegs G, Hentschel J, Wendel A. A. T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 1992;90:196-203. [Crossref] [PubMed]
- Cheng MH, Anderson MS. Monogenic autoimmunity. Annu Rev Immunol 2012;30:393-427. [Crossref] [PubMed]
- Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther 2012;36:517-33. [Crossref] [PubMed]
- Liberal R, Grant CR, Longhi MS, et al. Regulatory T cells: Mechanisms of suppression and impairment in autoimmune liver disease. IUBMB Life 2015;67:88-97. [Crossref] [PubMed]
- Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol Rev 2013;252:139-45. [Crossref] [PubMed]
- Keohane C, Kordasti S, Seidl T, et al. JAK inhibition induces silencing of T Helper cytokine secretion and a profound reduction in T regulatory cells. Br J Haematol 2015;171:60-73. [Crossref] [PubMed]
- Harusato A, Abo H, Ngo VL, et al. IL-36gamma signaling controls the induced regulatory T cell-Th9 cell balance via NFkappaB activation and STAT transcription factors. Mucosal Immunol 2017;10:1455-67. [Crossref] [PubMed]


