Prognostic factors for laryngeal sarcoma and nomogram development for prediction: a retrospective study based on SEER database
Introduction
Primary sarcoma of the larynx is rare, less than 1% of all larynx malignancies comprising less than 1% of laryngeal neoplasms in adults (1,2). Laryngeal sarcoma can arise from bone, cartilage, muscle, lipomatous, neuronal, and connective tissue, and is most commonly seen in the form of laryngeal chondrosarcoma(3-6). Other sarcomas are scarce and usually reported as case reports or small series (3-8). The diagnosis of laryngeal sarcoma mainly depends on pathology. The prognosis of laryngeal sarcoma may differ vastly due to the anatomical location, pathology, age, gender, and physical condition of the patient. However, due to the small number of cases and the lack of evaluation of the clinicopathological features of patients with laryngeal sarcoma, there is no standard protocol for the treatment of this rare disease, and no specific analysis of prognostic factors associated with laryngeal sarcoma (9).
Since 1973, the US National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database has recorded the incidence rate, treatment methods, case number, and prognosis of cancer patients in some US states. Data of 381 patients with laryngeal sarcoma between 1998 and 2016 were collected from the SEER database. These data were then analyzed to explore the risk factors and prognostic factors of laryngeal sarcoma, and a nomogram to predict the survival risk of patients with laryngeal sarcoma was constructed, to guide clinical treatment and prognosis.
Methods
Data collection
The data of patients who were diagnosed with laryngeal sarcoma between 1998 and 2016 were obtained from the SEER database by SEER Stat software (The Surveillance, Epidemiology, and End Results program institute SEER*State software, version 8.3.5). Since any information in the SEER database does not require explicit consent from the patients, our study was not subject to the ethical approval requirements of the institutional review board.
Patient screening
The inclusion criteria for this study were as follows. (I) Patients with a pathological diagnosis of laryngeal sarcoma from 1998 to 2016. (II) Pathological findings supported the patient’s diagnosis; (III) the patient was diagnosed with a primary tumor with no distant transfer; (VI) age at diagnosis ≥18; and (V) follow-up information including diagnosis of age, race, gender, pathology, pathological grade, primary site, lymph node metastasis, surgical treatment, and other clinical information was available. Patients who met any of the following criteria were excluded from the study: (I) unknown demographic information, including diagnostic age, gender, and race; (II) unknown clinical information, including primary site, pathology, and pathological grade; (III) unknown surgical treatment; (IV) unknown survival time; (V) Unknown vital status; or (VI) multiple primary tumors. A total of 381 patients with laryngeal sarcoma who met the criteria were screened and collected.
Statistical methods
The database data was obtained by SEER Stat 8.3.5 software, sorted by Excel 2016 software, and analyzed by SPSS (v25.0). The Kaplan-Meier curve was drawn for survival analysis. The variables obtained from the univariate analysis were introduced into the Cox proportional hazards model for multivariate analysis, to determine the risk factors affecting the prognosis of laryngeal sarcoma. Statistical significance was considered to exist when P<0.05.
Results
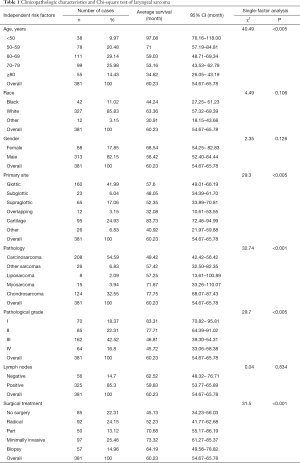
A total of 381 patients diagnosed with laryngeal sarcoma were included in the study. Most of the patients were white (85.83%) and male (82.15%). The majority (75.58%) were aged between 50 and 80 years old, with a median age of 67 years old (range, 20 to 85+ years). The primary site of most tumors was in the glottis (41.99%), followed by the laryngeal cartilage (24.93%). The most common pathology was carcinosarcoma (54.59%); and the second was chondrosarcoma (32.55%). Most patients presented with pathological grades III (42.52%) and II (22.31%) at the time of diagnosis. In most cases, metastatic lymph nodes were present (85.30%). In terms of treatment, the proportion of patients who had received surgical treatment was 62.73%, while 22.31% of patients had received no surgery, and 14.96% of patients had only undergone tissue biopsy. The specific circumstances are shown in Table 1.
Full table
Univariate analysis of prognostic factors
According to the survival rate statistics, 381 patients with laryngeal sarcoma were included in the study. The median age of diagnosis was 67 years, and the median survival time was 102.35 months. The 1-, 3-, 5-, and 10-year survival rates of laryngeal sarcoma were 87%, 76%, 61%, and 45%, respectively. The mortality rate within one year is 13%. Age (χ2=40.49, P<0.005), primary site (χ2=29.30, P<0.005), pathology (χ2=32.74, P<0.001), pathological grade (χ2=29.70, P<0.005), and surgical treatment (χ2=31.5, P<0.001) were significantly correlated with patient survival time.
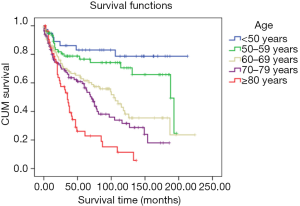
The survival status of laryngeal sarcoma patients in five age groups was inversely proportional, and was better for patients under 50 years of age (mean survival: 97.08 months, 95% CI: 76.16–118.00 months). Prognosis was not optimistic for patients older than 80 years of age (mean survival: 34.62 months, 95% CI: 26.05–43.19 months) (Figure 1).
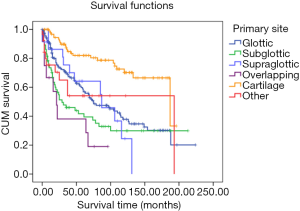
The survival time of different primary sites varied. Patients with laryngeal cartilage had the longest survival time, and patients with overlapping lesions of laryngeal sarcoma had the shortest. The group with the tumor originating in cartilage (mean survival: 83.73 months, 95% CI: 72.46–94.99 months) had statistical difference with the other groups (glottic vs. subglottic vs. overlapping vs. other; mean survival: 57.60 vs. 52.35 vs. 32.08 vs. 40.92 months; 95% CI: 49.01–66.19 vs. 33.89–70.81 vs. 10.61–53.55 vs. 21.97–59.88 months, P<0.05) except the supraglottic group (mean survival: 52.35 months, 95% CI: 33.89–70.81 months) (Figure 2).
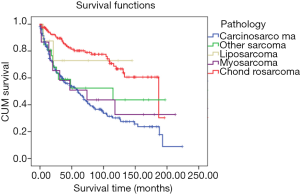
The patients with chondrosarcoma pathology had the best prognosis (mean survival: 77.75 months, 95% CI: 68.07–87.43 months). The mean survival time of patients with carcinosarcoma was the shortest of the five pathologies (mean survival: 49.42 months, 95% CI: 42.42–56.42 months). There was a statistical difference in the survival rate between chondrosarcoma and carcinosarcoma (P<0.001), but none between other pathology types (other sarcomas vs. liposarcoma vs. myosarcoma; mean survival: 57.42 vs. 57.25 vs. 71.67 months; 95% CI: 32.50–82.35 vs. 13.61–100.89 vs. 33.26–110.07 months) (Figure 3).
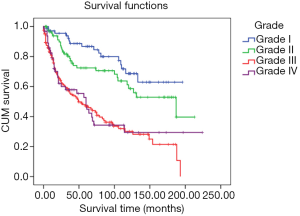
The pathological grade I group had the best prognosis (mean survival: 83.31 months, 95% CI: 70.82–95.81 months) was the best, followed by the grade II group (mean survival: 77.71 months, 95% CI: 64.39–91.02 months). Most patients had been diagnosed with grade III (proportion: 42.52%, mean survival: 46.81 months, 95% CI: 39.30–54.31 months). Patients in grade IV had the worst prognosis (mean survival: 45.72 months, 95% CI: 33.06–58.38 months) (Figure 4).
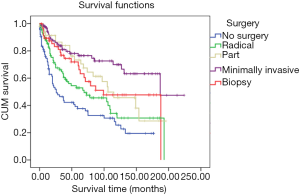
There was no statistical difference in prognosis between those patients who had received radical surgery, part surgery, and biopsy (radical surgery mean survival: 52.23 months, 95% CI: 41.77–62.68 months, part surgery mean survival: 70.68 months, 95% CI: 55.17–86.19 months, biopsy mean survival: 64.19 months, 95% CI: 49.56–78.82 months). However, a significant statistical difference was found between patients who had not received surgery and those who had undergone minimally invasive surgery (no surgery mean survival: 45.13 months, 95% CI: 34.23–56.03 months; minimally invasive surgery mean survival: 73.32 months, 95% CI: 61.27–85.37 months, P=0.01) (Figure 5).
Report on negative results
This study was based on survival analysis of 381 real events. Differences in survival based on race (χ2=4.49, P=0.106), gender (χ2=2.35, P=0.126), and, especially lymph node metastasis (χ2=0.04, P=0.834) were small and had no statistical difference.
Multivariate analysis prognostic factors
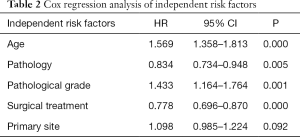
The factors obtained by univariate analysis were introduced into the Cox proportional hazards model for multivariate analysis. Age (HR: 1.599, 95% CI: 1.389–1.841), pathology (HR: 0.834, 95% CI: 0.734–0.948), pathological grade (HR: 1.693, 95% CI: 1.417–2.022), and surgical treatment (HR: 0.674, 95% CI: 0.575–0.790) were found to be independent risk factors affecting the survival of patients with laryngeal sarcoma (Table 2).
Full table
Prediction model nomogram development and verification
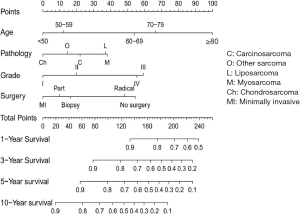
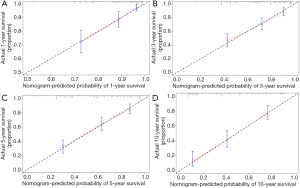
A nomogram was constructed based on the results of multivariate analyses and the accelerated failure time model. A weighted total score calculated from each variable was used to estimate the 1-, 3-, 5-and 10-year overall survival prediction (Figure 6). The nomogram was internally validated by discrimination and calibration methods. C-index was calculated as 0.73, which showed the excellent discrimination ability of the nomogram. The calibration plots showed a correlation between observed OS and nomogram-predicted OS (Figure 7).
Discussion
Epidemiological characteristics of laryngeal sarcoma
Sarcoma is a malignant tumor of mesoderm tissue, and laryngeal sarcoma is an extremely rare malignant tumor of the larynx, it accounts for only a small fraction of malignancies of the head and neck (far less than 1%) (1,2). Laryngeal sarcoma mostly occurs in adults, in the age range from 50 to 79 years and has a significantly higher incidence in males than in females (3,10). Previous studies suggested that alcohol addiction, smoking, and radiation exposure are more likely the major risk factors for laryngeal sarcoma (11,12). The symptoms are related to the primary site and adjacent structures of the tumor, and patients often experience hoarseness at the first stage, dyspnea, and even suffocation at an advanced stage. If the tumor penetrates the lower pharynx or esophageal entrance, pharyngeal discomfort, and even dysphagia may occur (10). Endoscopic examination of the larynx, CT, or MRI examination can effectively evaluate the tumor size and invasion range. B-ultrasound is sensitive to the detection of metastatic lymph nodes in the neck, and the PET test is often used to detect distant metastases. Earlier literature has reported that only 10–12% of patients with soft tissue sarcoma of the head and neck have cervical lymph node metastasis (13). However, in this study, 325 of the 381 cases (accounting for as much as 85.3%) were accompanied by lymph node metastasis. Diagnosis of laryngeal sarcoma requires histopathological examination, including HE staining, light microscopy, and immunohistochemical examination, to find the types of sarcomas (13). It should be emphasized that the pathological diagnosis of sarcoma needs to be approached with caution; the tumor tissue needs to be completely removed and multiple examinations need to be carried out. Typical pathological morphology and dependable immunohistochemical detection are needed, which should be combined with strict evaluation criteria to avoid misdiagnosis and missed diagnosis. Because laryngeal sarcoma is more rare, there is no standard treatment for this rare disease. Currently, surgery is the main treatment for primary laryngeal sarcoma. However, there are many pathological types of primary laryngeal sarcoma, whether radiotherapy and chemotherapy is an optional treatment is still controversial (9,12)
Laryngeal carcinosarcoma, also known as laryngeal spindle cell carcinoma or sarcomatoid cancer, is an extremely rare malignant tumor of the larynx. It represents only 2–3% of throat cancers, and the majority (about 93%) of patients are male (4,12,14). Studies suggested that alcohol addiction, smoking, and radiation exposure are more than likely the significant risk factors for laryngeal carcinosarcoma, and approximately 87% and 48% of these patients had a history of smoking and drinking, respectively (5,12). The most common site of primary laryngeal carcinosarcoma is the vocal cord, and so most patients experience hoarseness, and, at the advanced stage, some patients present with shortness of breath as the primary symptom. The associated symptoms included foreign body sensation in the throat, sore throat, and neck masses (4,12). There are epithelial cancer (adenocarcinoma or squamous cell carcinoma) and sarcoma (striated muscle, bone, cartilage, or fibrous, etc.) in the lesions of carcinosarcoma (13,14). The main treatment of carcinosarcoma is surgical resection. Currently, whether radiotherapy is an optional treatment for primary laryngeal carcinosarcoma is still controversial (12,15). Cao et al. (16) reported that tumor size, age, gender, and pathological grade are related to the prognosis of laryngeal carcinosarcoma. In our study, the 5-year survival rate of carcinosarcoma was 59%, and the median survival time was 76.95 months. The survival rate of patients with carcinosarcoma between no surgery and minimally invasive surgery had a statistical difference (P=0.046).
Chondrosarcoma is the most common mesenchymal tumor in the larynx, accounting for 0.007–2% of all laryngeal malignancies (3,17). Chondrosarcoma of the larynx is most often seen between the ages of 50 and 79 years and predominantly affects males. It usually originates from cricoid cartilage and the tumors are commonly found at the posterior lamina of cricoid cartilage (18,19). The symptoms last for a long time, and range from being tolerable in nature to dyspnea (4,19). The tumors grow slowly and are less invasive than other areas, and can stay in local infiltration for many years. Therefore, it is necessary to pay close attention to CT examinations for vocal cord paralysis of unknown cause. About 80% of laryngeal chondrosarcoma has calcifications that can be seen on CT (20). Pathologically, chondrosarcoma is classified into grades I, II, and III based on mitotic rate, cellularity, and nuclear size. Two histological variants have been described: clear cell, and undifferentiated, which is associated with worse prognosis (21). Based on most chondrosarcomas are low or intermediate grade (>95%) (22) conservative surgery is recommended as the initial treatment, and the treatment should be individualized based on the size and location of the lesion as well as the general conditions and age of the patient. Radical treatment is recommended for high-grade (17) and large, invasive tumors (23) for which conservative surgery would destabilize the cricoid ring (24). Radiotherapy is reserved for extensive lesions, inoperable cases, and recurrence (25). The prognosis is favorable with a specific disease survival at 1, 5, and 10 years of 97.7%, 91.4%, and 81.8%; the corresponding survival rates in our patients were 99%, 82%, and 72%. Despite the high rates of survival, local recurrence rates can reach up to 40% (17), and so regular review is needed after the surgery.
Laryngeal liposarcoma is rare, and only 8 cases were included in our study (24,26,27). It often occurs in men aged 40 to 60 years. The majority of laryngeal liposarcoma (75%) is supraglottic (26). Liposarcoma has four histopathological subtypes: well-differentiated, myxoid/round cell, dedifferentiated, and pleomorphic. Its tissue morphology is very similar to lipoma and can be distinguished by multicellular, nuclear atypical, infiltrating, and adipocytes. At the same time, positive staining with MDM2 and CDK4 is suggestive of liposarcoma (28). Liposarcoma of the head and neck is usually a low-grade tumor, but it can behave aggressively locally and recur (26,28). It is often diagnosed as liposarcoma after multiple relapses. So, treatment comprising thorough surgical removal, and radiotherapy after surgery should not be taken generally (26). Zhu reported that the overall prognosis was excellent, with 5-year survival up to 100% (7). However, the 5-year survival rate of laryngeal liposarcoma was only 64% in our study. This rate can be attributed to significant differences in survival rates because of the small number of cases enrolled in each study and the differences in age, pathological grade, and surgical treatment of the cases.
Myosarcoma includes leiomyosarcoma and rhabdomyosarcoma, with the former being more common. In this article, 11 of the 15 myosarcoma cases are leiomyosarcoma. Laryngeal leiomyosarcoma is mostly seen in adults, and there is a male predominance. It can occur in any part of the larynx, but supraglottic lesions are more often reported (29). Positive immunohistochemical staining with smooth muscle actin (SMA), h-caldesmon, muscle-specific actin (MSA) is used in the diagnosis (30). Surgery is the primary treatment for laryngeal leiomyosarcoma (31). Surgery performed with wide surgical margins, and tumor-free margins provide the best prognosis (32,33). In the recurrence or residual disease, radiotherapy is an adjuvant therapeutic modality, and chemotherapy may also have a limited role (34); Rhabdomyosarcoma has pleomorphic, acinar, embryonic, and grape-like histological characteristics, some scholars believe that the latter type is embryonic. Younger people are embryonic, and older people are mostly polymorphic (35). Histological characteristics are streaks or cytoplasmic myoglobin positive found in the cytoplasm of tumor cells (36). The primary treatment is surgical resection. The survival rate has increased markedly, owing to the improvement of adjuvant radiotherapy and chemotherapy (37,38). In children with localized disease, the 5-year survival with current multimodality treatment protocols exceeds 70% (37,38).
Other sarcomas of the larynx include fibrosarcoma, which is composed of fibroblasts arranged in fibrous bundles, fusiform, and nucleus in an oval shape (39). A pure fibrosarcoma is epithelial, and various mesenchymal tumor markers are negative, except for Vimentin (40). The primary treatment method is surgical resection with proper marginal tissue. The roles of chemotherapy and radiotherapy are not clear. The 5-year survival rates for highly differentiated and poorly differentiated fibrosarcoma are 50% and 5%, respectively (41). Laryngeal synovial sarcoma is more common in young men (42,43). The histological feature is bidirectional differentiation of the tumor; that is, both spindle cell sarcoma-like and adenoid-like components lining epithelial cells (44). Radical surgical resection with a negative margin is the preferred treatment. Neck dissection is not recommended because the tumors do not involve the lymph nodes. When the tumor involves the surgical margin, adjuvant radiotherapy, or chemotherapy can be given, and the radiotherapy is effective (42,44). Disease recurrence is a significant problem, with up to 45% of patients. The 2-, 5-, and 10-year DSS rates are 97%, 79% and 68% respectively (45,46); Angiosarcoma originates from the endothelium, has a nodular appearance, and is rich in blood vessels, or has an unclear boundary. A few tumors are solid, and the cells are spindle-shaped, like other sarcomas. The symptoms of angiosarcoma are not visible; it progresses rapidly and has high malignancy, often accompanied by hematogenous metastases. Surgery is the primary treatment, and radiotherapy can be added (47); malignant fibrous histiocytoma (MFH) subtypes are polymorphic, fibrous, giant cell, myxoid and inflammatory, and hemangioma-like. The prominent feature of MFH is the radial arrangement of the car. Masson, PTAH, and Mesh dyeing can help to find MFH.
Surgical treatment is the primary treatment method. The margin of the tumor is appropriately removed, but neck dissection is not recommended. Tumors are not sensitive to radiotherapy and chemotherapy (48,49); osteosarcoma is the smallest of laryngeal sarcoma (50,51) that occur in older men, and radiotherapy may be one of the causes (52). Imaging examination shows that the tumor is a soft tissue mass with invasive and destructive growth accompanied by calcification, which can also be an expansive lesion. The appearance of the tumor is polyp-like, and the texture is solid. The histological characteristics of osteosarcoma are that the tumor cells directly produce bone-like tissue or non-lamellar bone (12,53). Osteosarcoma has a high degree of malignancy, which can spread blood in the early stage and often metastasize to the lung (54). Radical resection is the first choice. Radiation alone is not enough, and adjuvant chemotherapy can be used (41). The 5-year survival rate is less than 50%, and the cut margin is an important indicator affecting the prognosis (55).
To sum up, the pathology of laryngeal sarcoma is important because it encompasses a broad range of types and with a wide differential diagnosis of sarcomas. Because the larynx is an inaccessible anatomic site, small fragmented surgical biopsies or crushed tissue may reveal little diagnostic tissue that sometimes results in diagnostic problems. A correct histologic diagnosis, classification, and grade of laryngeal sarcoma are vital important clinically. It has an impact on the prognosis of patients and the choice of treatment modalities by clinicians; for example, what kind of surgery should be done and whether it should be adjuvant to chemoradiation. For early laryngeal sarcoma, partial laryngectomy is an option, and keeping laryngeal function can improve the quality of life of patients. Minimally invasive surgical treatment has the advantages of less damage and fast recovery and has been increasingly accepted. Advanced laryngeal resection is recommended to avoid tumor recurrence. For early-stage laryngeal chondrosarcoma, conservative surgery is recommended (22). Liposarcoma should be completely removed to prevent recurrence (26). Synovial sarcoma (42,44), carcinosarcoma (12,15), osteosarcoma, and MFH (48,49) should be resected, to ensure clean margins. Meanwhile synovial sarcoma and MFH are not recommended to be treated with cervical lymph node dissection (42-44,48,49). Radiotherapy and chemotherapy have a certain effect on advanced laryngeal chondrosarcoma (24,25), osteosarcoma (55), leiomyosarcoma (37,38), angiosarcoma (47), and synovial sarcoma (42,44), and the margins are not clean. However, the effect they have on fibrosarcoma (50) and carcinosarcoma (12,15) is not clear. Radiotherapy is not recommended for treating MFH and liposarcoma (48,49).
Prognostic factors affecting laryngeal sarcoma
This study showed that among the explored risk factors, only the age, pathology pathological grade, and surgical treatment had a statistical impact on the prognosis of patients. A prognostic nomogram based on this was developed, which could be used to predict the probability of survival assessment (56). It is worth noting that the effect of lymph node metastasis on survival of laryngeal sarcoma was not statistically significant, which was clinically different from laryngeal cancer (57). Each influencing factor was scored, and each score was added to obtain a total score, according to the contribution degree of each influencing factor to the outcome variable in the model. Finally, the predicted value of the individual outcome event was calculated by a function conversion relationship between the total score and the probability of occurrence of the event. Based on the nomogram, we know that age has the most significant effect on the survival time of patients. As the age increased, the score was assigned a higher value, and the worse prognosis the patient had; the scores of pathological grade III and IV were significantly higher than those of grade I and II. Our nomogram found that the higher the pathological grade, the worse the prognosis is; this is consistent with literature reports (16). Regarding surgical treatments, the data summarized in this article found that those who had not undergone surgery had the highest score, and those who had received minimally invasive surgery had the lowest score; the survival rate between them was statistically significant (P<0.05). This may suggest that the treatment of laryngeal sarcoma should be based on surgery (2), primarily minimally invasive surgery should be paid more attention and adopted. However, we also found that patients who had received radical surgery also had higher scores. Considering that the patients who had undergone this type of surgery had advanced tumors, their prognosis was still poor, even with radical surgery; Patients with different pathology had different scores and prognosis. Chondrosarcoma had the lowest score, and liposarcoma and myosarcoma scored slightly higher than the others. This result is different from the earlier statistical results of clinicopathological characteristics and other studies (7,37,38). The small number of cases enrolled in each study and the differences in age, pathological grade, and surgical treatment of the cases may have led to this result. We will continue to follow up on the data and update the research, reduce the errors, and obtain more correct statistics. In a clinical context, four prognostic factors scores of age, pathology, pathological grade, and surgical method of a specific case are added together, the percentage corresponding to the total score is the estimated survival rate, so the nomogram can be used to predict patient prognosis in the future. The accuracy of the partial prediction model is better than that of the TNM staging system (58,59), which can help clinicians better determine patient status and help doctors make appropriate treatment strategies to improve treatment efficiency.
Insufficient and limited
Although the laryngeal sarcoma sample size of this report has been the largest so far and establishes a reliable nomogram for prediction, there are still several deficiencies that should be considered when interpreting our results. First, we excluded some patients because of the lack of data on relevant variables, including ethnicity, primary site, pathological grade, surgery, etc. Some subgroups had a small sample size, and part of the follow-up data was seriously missing. For example, there were only 8 and 15 patients with liposarcoma and myosarcoma. This disparity may have caused a deviation in the nomogram. Second, the SEER database does not record genetic factors and some interventions, including family history, genomic status, weight, and smoking, which may improve the predictive power of the nomogram. Third, although chemotherapy, radiotherapy, and targeted therapy are documented in the SEER database, it is not recommended for the construction of nomogram due to the incompleteness of the data and the bias caused by the patient’s willingness to treat. Fourth, a nomogram is subject to retrospective limitations on data collection, so prospective cohort studies must validate it before it can be used in clinical practice (60).
Acknowledgments
Funding: This project is supported by the Wu Jieping Medical Foundation (320.6750.19021) and China medical university young backbone support program (QGZ2018011).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2970.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2970). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Since any information in the SEER database does not require explicit consent from the patients, our study was not subject to the ethical approval requirements of the institutional review board. Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin HW, Bhattacharyya N. Staging and survival analysis for nonsquamous cell carcinomas of the larynx. Laryngoscope 2008;118:1003-13. [Crossref] [PubMed]
- Spector ME, Rosko AJ, Birkeland AC. Challenges in addressing early stage laryngeal squamous cell carcinoma. Transl Cancer Res 2018;7:1338-40. [Crossref]
- Karatayli-Ozgursoy S, Bishop JA, Hillel AT, et al. Non-epithelial tumors of the larynx: a single institution review. Am J Otolaryngol 2016;37:279-85. [Crossref] [PubMed]
- Thompson LD, Gannon FH. Chondrosarcoma of the larynx: a clinicopathologic study of 111 cases with a review of the literature. Am J Surg Pathol 2002;26:836-51. [Crossref] [PubMed]
- Luna-Ortiz K, Navarro-Santiesteban S, Villavicencio-Valencia V, et al. Primary laryngeal sarcomas in a Mexican population: Case series of eleven cases. Clin Otolaryngol 2017;42:1389-92. [Crossref] [PubMed]
- Li F, Wu Y, Chen L, et al. Evaluation of clinical risk factors for predicting insidious right central and posterior right recurrent laryngeal nerve lymph node metastasis in papillary thyroid microcarcinoma patients (cN0): experience of a single center. Ann Transl Med 2019;7:8. [Crossref] [PubMed]
- Zhu H, Sun J, Wei S, et al. Well-Differentiated Laryngeal/Hypopharyngeal Liposarcoma in the MDM2 Era Report of Three Cases and Literature Review. Head Neck Pathol 2017;11:146-51. [Crossref] [PubMed]
- Leventhal DD, Spiegel J, Keane W. Laryngeal alveolar rhabdomyosarcoma involving the true vocal fold in an adult: Case report. Ear Nose Throat J 2010;89:E8. [Crossref] [PubMed]
- Zhang Y, Huang Z, Gross N, et al. Multimodality Treatment Options and Outcomes of Laryngeal Carcinosarcoma: A Clinical Analysis of a Rare Tumor from a Single Hospital. Biomed Res Int 2019;2019:1754675.
- Saraydaroglu O, Narter S, Ozsen M, et al. Non-epithelial tumors of the larynx: case series of 12 years. Eur Arch Otorhinolaryngol 2019;276:2843-7. [Crossref] [PubMed]
- Thompson LD, Wieneke JA, Miettinen M, et al. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol 2002;26:153-70. [Crossref] [PubMed]
- Zhang M, Zhao LM, Li XM, et al. True carcinosarcoma of the larynx. J Laryngol Otol 2013;127:100-3. [Crossref] [PubMed]
- Wick MR, Swanson PE. Carcinosarcomas: current perspectives and an historical review of nosological concepts. Semin Diagn Pathol 1993;10:118-27. [PubMed]
- Ernster JA, Franquemont DW, Sweeney JP. Initial report of a case of carcinosarcoma of the supraglottis. Ear Nose Throat J 2000;79:384-7. [Crossref] [PubMed]
- Ballo MT, Garden AS, El-Naggar AK, et al. Radiation therapy for early stage (T1-T2) sarcomatoid carcinoma of true vocal cords: outcomes and patterns of failure. Laryngoscope 1998;108:760-3. [Crossref] [PubMed]
- Cao X, Liu J, Zheng Y, et al. Simultaneous squamous cell carcinoma with primary malignant fibrous histiocytoma of the larynx: a case report. Mol Med Rep 2012;5:971-3. [Crossref] [PubMed]
- Chin OY, Dubal PM, Sheikh AB, et al. Laryngeal chondrosarcoma: A systematic review of 592 cases. Laryngoscope 2017;127:430-9. [Crossref] [PubMed]
- Wang Q, Chen H, Zhou S. Chondrosarcoma of the larynx: report of two cases and review of the literature. Int J Clin Exp Pathol 2015;8:2068-73. [PubMed]
- Baatenburg de Jong RJ, van Lent S, Hogendoorn PC. Chondroma and chondrosarcoma of the larynx. Curr Opin Otolaryngol Head Neck Surg 2004;12:98-105. [Crossref] [PubMed]
- Casiraghi O, Martinez-Madrigal F, Pineda-Daboin K, et al. Chondroid tumors of the larynx: a clinicopathologic study of 19 cases, including two dedifferentiated chondrosarcomas. Ann Diagn Pathol 2004;8:189-97. [Crossref] [PubMed]
- Sauter A, Bersch C, Lambert KL, et al. Chondrosarcoma of the larynx and review of the literature. Anticancer Res 2007;27:2925-9. [PubMed]
- Piazza C, Paderno A, Nicolai P. Conservative surgery for laryngeal chondrosarcoma: a review of the most recently proposed approaches. Curr Opin Otolaryngol Head Neck Surg 2017;25:93-100. [Crossref] [PubMed]
- Piazza C, Del BF, Grazioli P, et al. Organ preservation surgery for low- and intermediate-grade laryngeal chondrosarcomas: analysis of 16 cases. Laryngoscope 2014;124:907-12. [Crossref] [PubMed]
- Esclamado RM, Disher MJ, Ditto JL, et al. Laryngeal liposarcoma. Arch Otolaryngol Head Neck Surg 1994;120:422-6. [Crossref] [PubMed]
- Oliveira JF, Branquinho FA, Monteiro AR, et al. Laryngeal chondrosarcoma--ten years of experience. Braz J Otorhinolaryngol 2014;80:354-8. [Crossref] [PubMed]
- Han Y, Yang LH, Liu TT, et al. Liposarcoma of the larynx: report of a case and review of literature. Int J Clin Exp Pathol 2015;8:1068-72. [PubMed]
- Pajaniappane A, Farzan J, Green DM, et al. Well-differentiated liposarcoma of the epiglottis. J Laryngol Otol 2014;128:296-8. [Crossref] [PubMed]
- Kodiyan J, Rudman JR, Rosow DE, et al. Lipoma and liposarcoma of the larynx: case reports and literature review. Am J Otolaryngol 2015;36:611-5. [Crossref] [PubMed]
- Selçuk ÖT, Renda L, Erol B, et al. A case of laryngeal leiomyosarcoma and review of the literature. Ann Maxillofac Surg 2015;5:274-6. [Crossref] [PubMed]
- Zambo I, Veselý K. WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol 2014;50:64-70.
- Morera Serna E, Pérez FCA, de la Fuente Jambrina C, et al. (Laryngeal leiomyosarcoma). Acta Otorrinolaringol Esp 2007;58:445-8. [Crossref] [PubMed]
- Abbas A, Ikram M, Yaqoob N. Leiomyosarcoma of the larynx: A case report. Ear Nose Throat J 2005;84:435-6, 440. [Crossref] [PubMed]
- Darouassi Y, Bouaity B, Zalagh M, et al. Laryngeal leiomyosarcoma. B-ENT 2005;1:145-9. [PubMed]
- Khadivi E, Taziky MH, Jafarian AH, et al. Laryngeal leiomyosarcoma, a case report and review of articles. Iran J Otorhinolaryngol 2013;25:253-8. [PubMed]
- Dikbas O, Altundag K, Abali H, et al. Embryonal rhabdomyosarcoma of the larynx. Otolaryngol Head Neck Surg 2005;133:160-2. [Crossref] [PubMed]
- Bertheau P, Deboise A, De Roquancourt A, et al. Leiomyosarcoma of the larynx. Histological, immunohistochemical and ultrastructural study of a case with review of the literature. Ann Pathol 1991;11:122-7. [PubMed]
- Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol 1995;13:610-30. [Crossref] [PubMed]
- Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol 2001;19:3091-102. [Crossref] [PubMed]
- Ferlito A. Laryngeal fibrosarcoma: an over-diagnosed tumor. ORL J Otorhinolaryngol Relat Spec 1990;52:194-5. [Crossref] [PubMed]
- Cowan ML, Thompson LD, Leon ME, et al. Low-Grade Fibromyxoid Sarcoma of the Head and Neck: A Clinicopathologic Series and Review of the Literature. Head Neck Pathol 2016;10:161-6. [Crossref] [PubMed]
- Myssiorek D, Patel M, Wasserman P, et al. Osteosarcoma of the larynx. Ann Otol Rhinol Laryngol 1998;107:70-4. [Crossref] [PubMed]
- Madabhavi I, Bhardawa V, Modi M, et al. Primary synovial sarcoma (SS) of larynx: An unusual site. Oral Oncol 2018;79:80-2. [Crossref] [PubMed]
- Okcu MF, Munsell M, Treuner J, et al. Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol 2003;21:1602-11. [Crossref] [PubMed]
- Wushou A, Miao XC. Tumor size predicts prognosis of head and neck synovial cell sarcoma. Oncol Lett 2015;9:381-6. [Crossref] [PubMed]
- Harb WJ, Luna MA, Patel SR, et al. Survival in patients with synovial sarcoma of the head and neck: association with tumor location, size, and extension. Head Neck 2007;29:731-40. [Crossref] [PubMed]
- Owosho AA, Estilo CL, Rosen EB, et al. A clinicopathologic study on SS18 fusion positive head and neck synovial sarcomas. Oral Oncol 2017;66:46-51. [Crossref] [PubMed]
- Pisani P, Krengli M, Ramponi A, et al. Angiosarcoma of the hypopharynx. J Laryngol Otol 1994;108:905-8. [Crossref] [PubMed]
- Bilewicz R, Wierzchowska M, Burduk PK, et al. Malignant fibrohistiocytoma of the larynx. Otolaryngol Pol 2007;61:325-8. [Crossref] [PubMed]
- Ferlito A, Nicolai P, Recher G, et al. Primary laryngeal malignant fibrous histiocytoma: review of the literature and report of seven cases. Laryngoscope 1983;93:1351-8. [Crossref] [PubMed]
- Sanaat Z, Mohammady G, Esmaili H, et al. Osteosarcoma of the larynx. Arch Iran Med 2009;12:499-502. [PubMed]
- Bahl A, George P, Bhattacharyya T, et al. Osteosarcoma of larynx: A rare case report with review of literature. J Cancer Res Ther 2015;11:1038. [Crossref] [PubMed]
- Ulusan M, Yilmazer R, Ozluk Y, et al. Radiation-induced osteosarcoma of the larynx: case report and literature review. Ear Nose Throat J 2012;91:E22-5. [PubMed]
- Roy S, Purgina B, Seethala RR. Spindle cell carcinoma of the larynx with rhabdomyoblastic heterologous element: a rare form of divergent differentiation. Head Neck Pathol 2013;7:263-7. [Crossref] [PubMed]
- Topaloglu I, Işiksaçan V, Ulusoy S, et al. Osteosarcoma of the larynx. Otolaryngol Head Neck Surg 2004;131:789-90. [Crossref] [PubMed]
- Berge JK, Kapadia SB, Myers EN. Osteosarcoma of the larynx. Arch Otolaryngol Head Neck Surg 1998;124:207-10. [Crossref] [PubMed]
- Paulino Pereira NR, Mclaughlin L, Janssen SJ, et al. The SORG nomogram accurately predicts 3- and 12-months survival for operable spine metastatic disease: External validation. J Surg Oncol 2017;115:1019-27. [Crossref] [PubMed]
- Fletcher KT, Gal TJ, Ebelhar AJ, et al. Prognostic indicators and survival in salvage surgery for laryngeal cancer. Head Neck 2017;39:2021-6. [Crossref] [PubMed]
- Pan JJ, Ng WT, Zong JF, et al. Prognostic nomogram for refining the prognostication of the proposed 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016;122:3307-15.
- Muneoka Y, Akazawa K, Ishikawa T, et al. Nomogram for 5-year relapse-free survival of a patient with advanced gastric cancer after surgery. Int J Surg 2016;35:153-9. [Crossref] [PubMed]
- Kazem MA, Khan AU, Selvasekar CR. Validation of nomogram for disease free survival for colon cancer in UK population: A prospective cohort study. Int J Surg 2016;27:58-65. [Crossref] [PubMed]