Phased surgical treatment of barium enema-induced rectal injury and retention of barium in the pelvic floor space
Introduction
Barium enema is a common and safe technique for digestive tract imaging. There are very few reports on the entry of barium into an undesired site due to unexpected injuries and the consequent impact, evolution and treatment for the associated injuries (1,2). We adopted a 1-year protocol for treating a patient who suffered severe injury to the rectal wall when undergoing routine barium enema prior to the surgery for uterine prolapse, and achieved successful results. In this case, iatrogenic injury to the rectal wall was developed during the initial barium enema and additional bowel preparation yet undetected, and the patient underwent a second barium enema, in which a large amount of paste barium entered into the perirectal space and caused serious infection. Long-term drainage and flushing was applied to clean out the retained barium but in vain. Following emergent colostomy with local cleaning and drainage, we monitored the impact of the barium retention on the control of regional infection, tissue repair and pelvic organs for a longer period, and established a phased surgical protocol taking into account the rectal injuries and accompanying vaginal fistula, by which the patient was eventually cured. The risk factors of injuries, diagnostic process, impact and evolution of barium in the body, impact of infections on pelvic organ prolapse and experience in the surgical treatment are discussed in the present study as below.
Case report
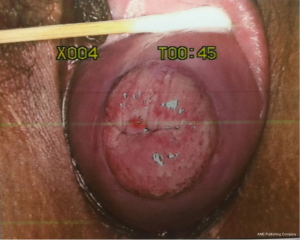
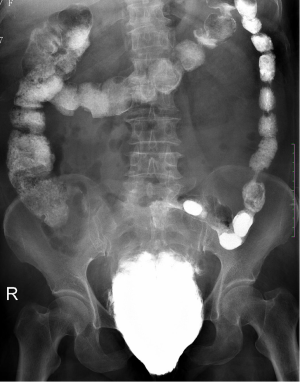
A 64-year-old woman was admitted to the department of gynecology in our hospital with mild anterior vaginal wall prolapse and IIº uterine prolapse (Figure 1). She had no medical history and no contraindication for surgery was found in the routine preoperative examinations. A barium defecography was scheduled for detailed assessment of prolapse of the pelvic organs. On the day of examination, the imaging was not successful due to accumulated feces in the rectum as a result of improper intestinal preparation. As the second test following soapsuds enema still failed, the patient returned to her ward. She developed lower abdominal pain that night with perineal and anal pain but without fever, and the posterior vaginal wall was mildly edematous and congestive. Associating the discomfort with the primary condition and repeated enemas, the gynecologist merely administered warm water bath and anal suppositories to reduce the mucosal inflammation. Later, the patient felt alleviated. Three days after the initial defecography, she underwent the third examination, in which 300 g of paste barium in saline was injected via the anus. Anal sphincter incontinence and bowel dysfunction were then noted by the imaging physician (Figure 2), which was confirmed as misdiagnosis in the subsequent treatment, for the barium had entered into the perirectal space at the time of injection. The patient developed gradually worsening lower abdominal and perineal pain. Two days after the imaging, she had a fever of 38 °C, blood leukocyte of 19.3×109/L and neutrophil ratio (NEUT %) of 89.9%, which increased to 20.3×109/L and 92.5%, respectively, in a repeat test. Swelling of the labia minora and perineum was noted in physical examination, and the rectal wall was edematous and tender by rectal touch. No barium retention was found in the rectum and significantly thickened rectovaginal septum was palpable through bimanual palpation. Emergent colonoscopy revealed stripped mucosal erosion of the anterior rectal wall 5-10 cm from the anus with a dirt surface and swollen adjacent mucosa. Pelvic radiography showed spindle barium aggregation with a maximum diameter of about 10 cm (Figure 3). The diagnosis was rectal anterior wall injury, perirectal space infection and barium retention.
On the same evening, the patient received laparoscopic exploration and transverse colostomy with catheter drainage of the perirectal space under general anesthesia. Mild edema of the peritoneal reflection was visible under laparoscopy. While there was no sign of involvement in the peritoneal cavity, the infection was confined to the pelvic cavity below the reflection. The swollen anterior rectal wall and mucosal injury were evidently palpable by digital rectal examination in the surgery. Debridement was easily completed with fingers, and about 300 mL of smelly, white paste barium was discharged with pus. A triangular cleft was then formed at the anterior rectal wall 4-10 cm from the anus with the tip inward, through which the rectovaginal septum and the perirectal space aside could be reached with a finger. After wound flushing through the anus, two small symmetrical incisions were made on the perineal skin for placing two drainage tubes at both sides of the space and a transanal drainage tube was placed also for postoperative flushing and drainage.
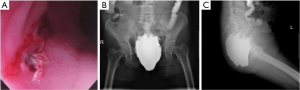
The patient was on antibiotics for one week postoperatively. Her temperature was back to normal four days later, and blood tests were normal one week after the surgery. Metronidazole 200 mL and plenty of normal saline were used to flush the perineal tube with drainage twice daily. The local swelling gradually subsided in 2 weeks after surgery as pus drainage (with a little barium) reduced and flushing fluid clear. Since the patient could not get out of bed to walk due to severe pain with the perineal catheters and transanal tubes, they were removed one after another in three weeks postoperatively. The wound was directly flushed through the anus with a soft tube. The perineal swelling and pain disappeared three weeks after surgery, and she resumed normal walking and other activities. The symptoms of uterine prolapse were gone and urinary function was normal. Upon digital rectal examination, the cleft on the anterior rectal wall did not change significantly, though the edges were hardened. The rectovaginal septum and both sides of the perirectal space could still be reached with a finger through the cleft. A repeat pelvic radiograph showed only a small reduction in the retained barium compared with the preoperative condition (Figure 4A). Flushing of the perirectal space was continued through the anus and the cleft on a daily basis, though only a small amount of smelly, purulent exudate was washed out with bloody fluid, and there was no barium in the flushed fluid. Six weeks after surgery, outflow of the flushing fluid from the vagina was noted. Examination revealed a fistula on the left posterior vaginal wall 4 cm from the vaginal orifice, surfaced with red granulation tissues. Pelvic radiographic examinations were repeated at weeks 5, 8, 15, 22 and 31 after surgery, which showed no change in the amount, morphology or scope of retained barium in the pelvic spindle-shaped area (Figure 4B-F).
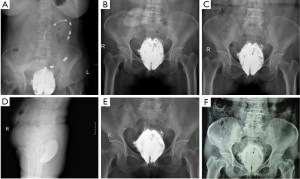
The patient continued daily transanal irrigation through week 31 after surgery. The fluid became cleaner with less blood and the smell gradually disappeared, so the frequency of flushing reduced from once daily to every 2 days. Upon digital rectal examination, the cleft on the anterior rectal wall was slightly narrowed with an irregular diamond shape of about 5 cm long and 3 cm wide. The distal end was around 3 cm from the dentate line, and the edges were significantly hardened. The rectovaginal septum and bilateral perirectal spaces could be reached by fingers, and bright red blood was observed on the glove. The vaginal fistula remained unimproved. The injuries on the anterior rectal wall and inflammation areas of the rectovaginal septum along with both sides of the perirectal space were evidently visible on MRI at week 31 after surgery (Figure 5A-C). Upon clinical assessment, healing of the injuries or the vaginal fistula was considered impossible in view of the large cleft on the anterior rectal wall with unmodifiable hardened scar tissues, and irremovable barium retained in the perirectal space. Given that the wound surface was clean and covered with granulation tissues after repeated flushing, a second surgery was planned for the patient.
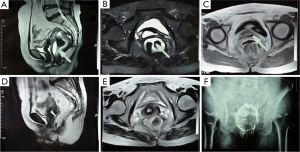
At week 32 after the first surgery, she received the second operation (phase II) under general anesthesia. The original transverse colon fistula was closed during the procedure. After ligation and interruption of the inferior mesenteric artery and vein, a portion of the rectum was resected from 1.5 cm above the dentate line to the peritoneal reflection. When the mesorectum were divided, a large quantity of white, solid substances were observed and removed. Plenty distilled water and 0.03% povidone-iodine solution were used to rinse the pelvic wound. The distal end of the colon was anastomosed on the dentate line, and terminal ileostomy was achieved at the end of surgery. Postoperative recovery was smooth except for incision infection, and wound healing was satisfactory after dressing changes. There was no liquid or gaseous discharge from the vagina after surgery. Two weeks postoperatively, MRI and pelvic radiography showed intact rectal wall in the pelvic cavity, with only a small amount of discontinuous inflammatory exudation around the rectum and much less barium compared with the condition before surgery (Figure 5D-F). Digital anal dilation was administered twice daily from one week after the operation, until the opening was as large as two fingers dilated. The patient did not experience discomfort except for intermittent mild constipation during the procedure.
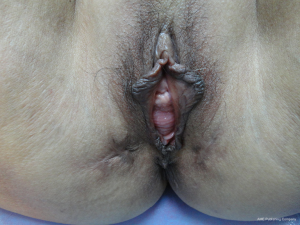
Three months after the second operation, the patient’s nutritional, physical and dietary conditions were normal upon assessment. She had intermittent mild constipation, which could be relieved by using lubricants. Urinary function was normal. No vaginal mass was discharged when abdominal pressure increased. She did not complain of any other discomfort. Therefore, the third operation (Phase III) was performed under general anesthesia to close the stoma at the terminal ileum. The patient recovered smoothly from the surgery and was discharged, with digital anal dilation continued on a daily basis. A 3-month follow up showed normal diet and bowel function, and she was free of abdominal or perineal discomfort. The anal dilation was up to two fingers without any palpable abnormality in the rectum. Gynecological examination revealed that the vaginal fistula had healed and uterine prolapse disappeared, though the mild anterior vaginal wall prolapse persisted (Figure 6).
Discussion
Although barium enema-induced colorectal injuries are extremely rare, underlying colorectal diseases may still precipitate some injuries during a routine barium enema. Serious peritonitis and sepsis, as well as intestinal adhesion and bowel obstruction in a long run, will be the result once the peritoneal cavity is affected by the injury and barium leakage. However, when only a portion of the rectum below the peritoneal reflection is involved, infection may be confined to the pelvic space with mild symptoms in the early stage, which is easily overlooked and may lead to delayed diagnosis. The consequences associated with the leakage of barium may also vary in the manifestation.
As shown by the colonoscopy in the case of this study, the anterior rectal wall of the patient was injured by the tip of the enema tube. In the absence of timely diagnosis, the following barium perfusion resulted in retention of barium agents as they entered and aggregated in the perirectal space through the wound. Although an overview of the rectal morphology before the injury was not available, the presence of internal rectal prolapse was believed to be one of the underlying causes. The pelvic floor weakness and long-term increased abdominal pressure, common in patients with uterine prolapse, are also risk factors of rectal prolapse. As the physician or nurse performing the enema did not give extra care to these factors, nor did they conduct a more careful digital rectal examination before the procedure, the patient’s rectal wall was injured by the tube tip during the routine operation.
In view of the symptoms of the patient, the rectal wall injury was supposed to form and progress gradually. Symptoms were not apparent after the initial injury, while there were only scratches on the rectal mucosa and the rectal wall was not penetrated. However, multiple enemas and anal suppositories were used despite the patient’s complaint, which did not only worsen the rectal laceration but also lead to spread of bacterial infection along the injured area, resulting in infection in the rectal wall and inflammation and edema of the rectovaginal septum, posterior vaginal wall and perirectal space. At the last barium enema, a large amount of barium broke through the injured rectal wall under increased pressure and entered the perirectal space. As the patient developed anal sphincter dysfunction, the residual barium in the rectum was discharged due to incontinence, while that in the perirectal space was retained and caused severe infection.
Upon the occurrence of perirectal space infections, colostomy and drainage of the affected area should be performed as an emergent treatment. In the present case, we conducted laparoscopic exploration to achieve colostomy so that the injury and involvement of the peritoneal cavity could be understood. Minimizing the abdominal wall injury and postoperative abdominal adhesions, laparoscopic surgery contributed to a more favorable environment for subsequent operations. In the event that barium contamination was detected in the peritoneal cavity, conversion to laparotomy would be inevitable for extensive cleaning and drainage. Approximately 300 mL of pyogenic fluid with barium was expelled by squeezing and debridement of the perirectal space with fingers, but repeated normal saline flushing did not contribute to obvious additional amount of excluded barium. A repeat pelvic radiograph showed only a small reduction in the retained barium compared with the preoperative condition, so the debridement only expelled the barium mixed with the purulence while the agents retained in the tissue were not washed out at all. Intraoperative digital examination and MRI later both showed that the infection was confined to the rectovaginal septum and both sides along the rectum, without involving the posterior region of the rectum.
The patient was under close monitoring for over 7 months following the initial emergent operation, because we could not identify the impact of regional flushing and drainage on the retained barium, as well as the potential change and impact on the patient of the retention. It was also uncertain whether the barium could be wrapped and expelled as a process of natural rejection, similar to that with suture knots from surgical wounds, or whether the distribution of retained barium would change in the tissue repair process. While barium was still washed out in the flushing fluid in the first two weeks after surgery, the amount was not large and even less later, and eventually disappeared in the subsequent flushing. In the following digital examinations, there was no barium trace on the blood-stained glove, suggesting that the barium powder had been wrapped in granulation tissues. The edges of the rectal wall laceration had gradually formed into a thick, hard scar contracture in seven months, while a similar process was not observed in the posterior vaginal wall adjacent to the barium-contaminated area. Repeated pelvic radiographies showed no change in the amount, morphology or scope of retained barium. In the phase II operation, the retained barium was partially removed as the inflammatory tissues around the rectum was resected, whereas radiograph showed no change in the distribution of the remaining barium in the peripheral tissues compared with the preoperative conditions. The imaging findings of that patient suggested that the barium powder was retained and fixed in the interstitial space without change of distribution for at least 35 weeks, which could not be removed by flushing alone. In the follow up visits for 4 months after the phase III operation, the patient was free of lower abdominal and perineal discomfort, and had normal urinary function. However, extended follow-up would be needed to determine the long-term evolution and impact on the body of the remaining barium.
The decision on the phase II surgical plan was mainly dependent on the rectal wall injury and the accompanying vaginal fistula. Approximately eight months after the initial surgery, the cleft on the rectal wall evolved into an irregular diamond shape of about 5 cm long and 3 cm wide, with significantly hardened edges, making repair in situ impossible. Hence, we decided to resect the injured rectum, anastomose the proximal end of the colon to the anal canal, and create an upstream colostomy. Since transverse colostomy was performed in the phase I surgery and the left colon was short, the stoma was closed in phase II to ensure minimized tension when the colon was dragged down for anastomosis. The terminal ileum was then used to form a stoma, which was closed three months later for smooth healing of both the anastomosis and the closed original colon stoma. The other significant determinant of the phase II operation was the cleanliness of the original wound surface with infection. The surgery could only be performed on a relatively clean wound surface to prevent postoperative pelvic infection, allowing successful healing of both the anastomosis and vaginal fistula. Odor pus had disappeared after long-term irrigation of the original infected area, leaving only a small amount of exudate. Blood-stained glove on digital examination suggested the growth of granulation tissues, which were relatively clean and suitable for the implementation of the second operation. While the injured rectum was removed, the infected regions around the rectum were debrided to remove both inflamed tissues and part of the residual barium. Postoperative MRI showed only a small amount of discontinuous inflammatory exudate around the original infection area. The dragged-down colonic anastomosis enabled complete cover and isolation of the vaginal fistula with the intestinal wall, facilitating the healing in a contamination-free environment. It was demonstrated that both the relative cleanness of the surgical area before operation and intraoperative debridement contributed to successful healing of the anastomosis and vaginal fistula.
One concern after coloanal anastomosis was potential difficulty in defecation as a result of anastomotic stenosis due to tissue scar contracture. Hence, daily anal dilation was critical as soon as anastomotic healing was achieved. After six months of continuous dilation, the anus could be dilated up to two fingers, and the patient’s intermittent mild constipation could be resolved through the use of lubricants and dietary adjustments.
The evolution of vaginal fistula and uterine prolapse in this patient was also worth noting. Following the initial injuries to the rectal wall, evident edema was noted of the posterior vaginal wall and rectovaginal septum, though no vaginal fistula was found from the phase I surgery to 6 weeks thereafter. The occurrence of vaginal fistula six weeks after the first operation should associate with perirectal infection, and the lesion was difficult to repair in the absence of a clean wound surface. In the phase II operation, the original wound was debrided and the fistula was covered and isolated with an intact intestinal wall, creating the conditions for self-healing. Later, the vaginal fistula healed automatically. Three weeks after the phase I surgery, the patient was ambulant and uterine prolapse had disappeared, which could be explained by regional tissue adhesion caused by excessive inflammatory exudate due to severe infections in the rectovaginal septum, where the lower segment of the posterior wall of the uterine was elevated and fixed (Figure 5C). Since the infection and inflammation were limited to the posterior vaginal wall, the anterior vaginal wall prolapse had persisted (Figure 6).
There are several aspects that should be given care to during the management of this patient. When carrying out barium enema for patients with pelvic floor organ prolapse, the risk of rectal injury should be observed in light of the changes in anatomic structures. Rectal injuries, if not involving the peritoneal cavity, may be ignored at the early stage because of the mild symptoms, and the patient’s chief complaint and the digital rectal examination should be regarded. Retained barium that can not be washed out or drained appears to have no adverse effect on tissue repair, infection control and bowel function, though further clinical data will be needed to confirm its long-term effects. Phased operational protocols should be designed based on the specific injuries to control the infection and salvage the intestinal structures and functions. During the inflammatory and repairing process after infections in the pelvic cavity, prolapse symptoms may disappear due to adhesion and elevation of the prolapsed organ.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- de Feiter PW, Soeters PB, Dejong CH. Rectal perforations after barium enema: a review. Dis Colon Rectum 2006;49:261-71. [PubMed]
- Williams SM, Harned RK. Recognition and prevention of barium enema complications. Curr Probl Diagn Radiol 1991;20:123-51. [PubMed]