Efficacy and safety of sofosbuvir-containing regimens in patients with chronic hepatitis C virus infection after liver transplantation: a meta-analysis
Introduction
Hepatitis C virus (HCV) infection is the leading indication for liver transplantation (LT) worldwide (1,2). In patients with whom the virus is detectable at the time of transplantation, the recurrence of HCV is universal and immediate (3,4). Aggressive clinical features often characterize recurrent HCV infection after LT, and patients can rapidly progress to cirrhosis, decompensation, and increased risk of morbidity and mortality (5-7). For patients with recurrent HCV infection after LT, the treatment options are complicated (8). Those treated with pegylated interferon (Peg-IFN) and ribavirin often experience low rates of sustained virologic response (SVR) and significant adverse effects (9). While triple treatments with protease inhibitors have slightly improved the efficacy, they exacerbate adverse events (AEs) (10). Therefore there is a great need for a regimen that is both more potent and more tolerable without drug interactions for LT patients with recurrent HCV infection (8).
The development of direct-acting antivirals (DAAs) for the treatment of HCV has been eagerly anticipated. SOF, an oral nucleotide analog inhibitor of the HCV non-structural 5B (NS5B) polymerase, has recently been approved for the treatment of HCV genotypes 1–4. As reported, SOF, in combination with other DAAs or ribavirin, has demonstrated excellent efficacy and low rates of AEs (11,12). To date, no study has carried out a comprehensive evaluation of the efficacy and safety of SOF-containing treatment for patients with recurrent HCV infection after LT. This study aims to summarize the currently available data on the treatment of recurrent HCV in patients after LT with SOF and to supply guidance in practical clinical algorithms.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3074).
Methods
Searching strategy and literature selection
The literature search and data collection were performed in January, 2018. Three electronic databases, including the PubMed, EMBASE, and Cochrane Library databases, were searched. The literature search was performed using the following terms: “hepatitis C” or “HCV” or “hepacivir*”; “sofosbuvir” or “Sovaldi” or “SOF” or “GS-7977” or “PSI-7977”; “liver transplantation.” A manual search for potentially eligible studies was also performed by checking the references of the included studies and published narrative reviews.
We collected clinical trials, prospective cohort studies, or case-control studies that assessed the efficacy and safety of the SOF-containing regimens in patients with recurrent HCV infection after LT. Studies meeting the following criteria were included: (I) the study population were patients with recurrent HCV infection after LT; (II) the therapy included SOF; (III) the primary outcome was SVR12 (sustained virologic response 12 weeks) and the data of SVR12 was available; and (IV) the study showed safety-related outcomes. Studies were excluded if they met any of the following criteria: (I) the participants did not undergo LT; (II) the primary outcome measure was not SVR12, or the value of SVR was not available; or (III) conference abstracts without full text.
All the reviewing and screening processes in this study were based on the PRISMA guidelines for reporting systematic reviews. At each stage of the screening, we discussed the included and excluded studies, and resolved any discrepancies.
Data extraction
The following data were extracted from each study: first author’s name, publication time, study design (including sample size, drug dose, and treatment duration), patients’ clinical characteristics (including age, sex, HCV RNA level, and indications of liver function), and efficacy and safety outcomes.
The methodological quality of each study included in this analysis was assessed by the Jadad Scale (13), which has three items: randomization, blinding, and withdrawals and dropouts. The lowest score is 0, and the highest score is 2 for the first 2 items and 1 for the third item. The total score for each study can range from 0 to 5. The studies that scored 3 or more were considered to be of a higher quality.
Statistical analysis
All the analyses were performed using Stata (version 13.0). The statistically significant level was set at 0.05. Heterogeneity test was performed using and χ2 and I2 indices. If heterogeneity existed among the included studies, then a random effects model was used. Otherwise, a fixed effects model was adopted (14). Publication bias was assessed by Begg’s test, along with a funnel plot (15). Meta-regression analysis was also performed.
Results
Characteristics of the included studies
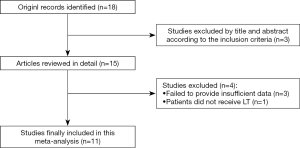
A total of 18 potentially relevant studies were found through the electronic searches. After a review of titles and abstracts, 15 articles were chosen for a full review. Finally, 11 articles (involving 12 studies) meeting the inclusion, and exclusion criteria were included in this meta-analysis (8,11,16-24). The selection process is shown in Figure 1.
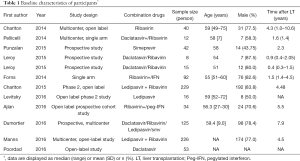
The selected papers involved 892 patients with recurrent HCV infection after LT who were treated with a SOF-containing regimen. The percentage of men in each study ranged from 33.3–87.5%. The median age of the patients ranged from 51–60 years. The baseline characteristics of the patients are shown in Tables 1 and 2.
Full table
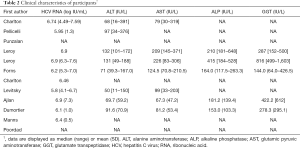
Full table
Efficacy outcome
The pooled analysis of SVR12 is shown in Figure 2A. A random effects model was adopted because the I2 was over 80% (χ2=62.21, P<0.01). Two studies were excluded, as all participants achieved SVR12. The pooled SVR12 was 88.1% (95% CI: 82.8–93.3%).
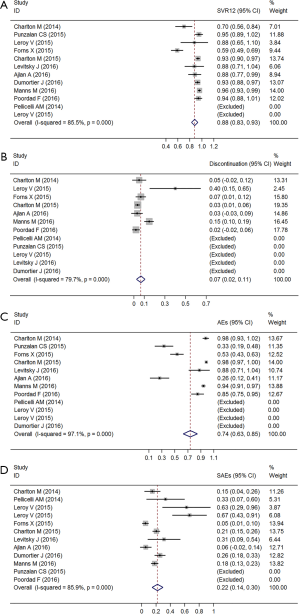
Safety outcomes
By pooling the data from 7 of the 12 studies, we found that the heterogeneity of incidence of discontinuation existed (χ2=29.54, P<0.01). The pooled estimate of the rate of discontinuation was 6.5% (95% CI: 2.4–10.7%) (Figure 2B). The heterogeneity of the incidence of AEs and serious adverse events (SAEs) also had statistical significance (χ2=239.02, P<0.01; χ2=63.89, P<0.01). The pooled estimates of the rate of AEs and SAEs were 73.7% (95% CI: 62.7–84.6%) and 22.1% (95% CI: 14.4–29.7%), respectively (Figure 2C,D).
The most common AEs were anemia, nausea, renal failure, aminotransferase increase, fatigue, and joint pain. Pooled results from 6 of the 7 studies revealed high heterogeneity.
Subgroup analysis and Meta-regression analysis
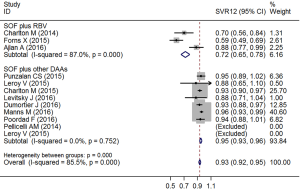
Subgroup analysis showed that patients treated with SOF plus other DAAs had higher SVR12 than those treated with SOF plus ribavirin or peg-IFN (χ2=43.35, P<0.01; Figure 3).
We performed a meta-regression analysis to explore the factors associated with the effect and safety outcomes. The data that was taken into consideration were: age, the proportion of men, time from liver transplantation (LT), the level of HCV RNA, alanine aminotransferase (ALT), alkaline phosphatase (ALP), glutamic pyruvic aminotransferase (AST), and glutamate transpeptidase (GGT). The result showed that the levels of ALT and AST were associated with the rate of SAEs (t=3.44, P=0.01; t=2.95, P=0.032).
Publication bias
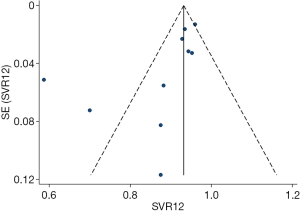
Begg’s test was performed to evaluate publication bias and revealed no statistical significance (z=1.43, P=0.15). The funnel plot for SVR12 is shown in Figure 4. The studies were distributed closely within the 95% confidence interval axis, which indicated no obvious publication bias.
Discussion
This meta-analysis evaluated the efficacy and safety of SOF for the treatment of patients with recurrent HCV infection after LT and found that the SVR12 was 88.1% (95% CI: 82.8–93.3%) and the rate of any AE was 73.7% (95% CI: 62.7–84.6%).
SOF is a potent NS5B nucleotide polymerase inhibitor with all HCV genotype activity and a high barrier of resistance (25). While it is currently an attractive target for anti-HCV treatment, the effect in patients who have received LT is still unclear. This study was the first study that aimed to evaluate a drug in post-transplant patients with recurrent HCV infection. Our findings show a potent efficacy and a favorable safety profile for SOF-containing regimens. Based on regression analysis, the efficacy of the regimens was not associated with valuable clinical indexes. Subgroup analysis of patients who received combination therapy found that treatment with SOF and other DAAs showed higher SVR12 than those treated with SOF and RBV or peg-IFN. Previous studies have shown that RBV is not needed when SOF and daclatasvir are used (16,26). Despite the increased rate of side effects and decreased effect with RBV, the possibility of shorter treatment time and reduced cost may lend support to the use of RBV, especially in patients for whom prior treatment has failed (27). However, a more extensive study is needed to confirm that RBV does not increase the efficacy in patients with recurrent HCV infection after LT.
Our study also showed a high SAE rate in this patient population. We performed regression analysis and found that the levels of ALT and AST were associated with the rate of SAEs, which indicates that the high SAEs were not related to the study drugs but rather to the underlying liver disease. These findings strongly suggest that post-LT patients should receive treatment early on and that use in advanced liver disease should be discouraged (17). Although the heterogeneity was significant, it can be accounted for by the different combination therapies used in subgroup analysis.
There are some limitations in this meta-analysis that should be considered. First, since our study only focused on post-transplant patients with HCV infection patients, we could not assess the efficacy and safety of SOF-containing regimens in patients with other advanced liver diseases. Second, single-arm trials were included; thus, we could not derive the absolute values of safety and efficacy indexes, and the present information was not sufficient to allow a precise subgroup analysis based on HCV genotype, level of disease, or treatment duration. Third, we did not conduct a cost-effective analysis to assess the benefit of the treatment regimen. Finally, as the number of included studies was small, more large-scale, and longer-term studies based on survival outcomes and the increasing safety profiles evaluating SOF-containing regimens will be of interest.
Conclusions
In summary, the present meta-analysis suggests that SOF-containing regimens have proved useful for the treatment of patients with recurrent HCV after LT. Compared to RBV or peg-IFN, patients treated with SOF combined with other DAAs have higher SVR12. In post-LT patients, treatment should be started early, and use in advanced liver disease should be taken into consideration. Further studies to investigate survival and safety outcomes for patients are needed.
Acknowledgments
The authors are very thankful to Mr. Tu for helpful consultation and comments on this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3074
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3074). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002;36:S30-4. [PubMed]
- Solli P, Dolci G, Ranieri VM. The new frontier of hepatitis C virus (HCV)-mismatched heart and lung transplantation. Ann Transl Med 2019;7:S279. [Crossref] [PubMed]
- Garcia-Retortillo M, Forns X, Feliu A, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology 2002;35:680. [Crossref] [PubMed]
- Wiesner RH, Sorrell M, Villamil F. Report of the First International Liver Transplantation Society Expert Panel Consensus Conference on Liver Transplantation and Hepatitis C. Liver Transpl 2003;9:S1-9. [Crossref] [PubMed]
- Forman LM, Lewis JD, Berlin JA, et al. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 2002;122:889-96. [Crossref] [PubMed]
- Berenguer M, Prieto M, Rayã N JM, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology 2000;32:852-8. [Crossref] [PubMed]
- Charlton M, Ruppert K, Belle SH, et al. Long-term results and modeling to predict outcomes in recipients with HCV infection: results of the NIDDK liver transplantation database. Liver Transpl 2004;10:1120-30. [Crossref] [PubMed]
- Forns X, Charlton M, Denning J, et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology 2015;61:1485-94. [Crossref] [PubMed]
- Lens S, Gambato M, Londoño MC, et al. Interferon-free regimens in the liver-transplant setting. Semin Liver Dis 2014;34:58-71. [Crossref] [PubMed]
- Coilly A, Roche B, Dumortier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol 2014;60:78-86. [Crossref] [PubMed]
- Charlton M, Gane E, Manns MP, et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 2015;148:108-17. [Crossref] [PubMed]
- Sulkowski MS, Gardiner DF, Rodrigueztorres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370:211-21. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [Crossref] [PubMed]
- Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998;316:469-author reply 470. [Crossref] [PubMed]
- Pellicelli AM, Montalbano M, Lionetti R, et al. Sofosbuvir plus daclatasvir for post-transplant recurrent hepatitis C: potent antiviral activity but no clinical benefit if treatment is given late. Dig Liver Dis 2014;46:923-7. [Crossref] [PubMed]
- Punzalan CS, Barry C, Zacharias I, et al. Sofosbuvir plus simeprevir treatment of recurrent genotype 1 hepatitis C after liver transplant. Clin Transplant 2015;29:1105-11. [Crossref] [PubMed]
- Leroy V, Dumortier J, Coilly A, et al. Efficacy of Sofosbuvir and Daclatasvir in Patients With Fibrosing Cholestatic Hepatitis C After Liver Transplantation. Clin Gastroenterol Hepatol 2015;13:1993-2001.e1-2.
- Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology 2015;149:649-59. [Crossref] [PubMed]
- Levitsky J, Verna EC, O'Leary JG, et al. Perioperative Ledipasvir-Sofosbuvir for HCV in Liver-Transplant Recipients. N Engl J Med 2016;375:2106-8. [Crossref] [PubMed]
- Ajlan A, Al-Jedai A, Elsiesy H, et al. Sofosbuvir-Based Therapy for Genotype 4 HCV Recurrence Post-Liver Transplant Treatment-Experienced Patients. Can J Gastroenterol Hepatol 2016;2016:2872371. [Crossref] [PubMed]
- Dumortier J, Leroy V, Duvoux C, et al. Sofosbuvir-based treatment of hepatitis C with severe fibrosis (METAVIR F3/F4) after liver transplantation. Liver Transpl 2016;22:1367-78. [Crossref] [PubMed]
- Manns M, Samuel D, Gane EJ, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016;16:685-97. [Crossref] [PubMed]
- Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 2016;63:1493-505. [Crossref] [PubMed]
- Sofia MJ, Bao D, Chang W, et al. Discovery of a β-d-2'-deoxy-2'-α-fluoro-2'-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. Journal of Medicinal Chemistry 2010;53:7202-18. [Crossref] [PubMed]
- Fontana RJ, Hughes EA, Bifano M, et al. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant 2013;13:1601-5. [Crossref] [PubMed]
- Tao T, Jiang X, Chen Y, et al. Efficacy and Safety of Ledipasvir/Sofosbuvir with and without Ribavirin in Patients with Chronic Hepatitis C Virus Genotype 1 Infection: a meta-analysis. Int J Infect Dis 2017;55:56-71. [Crossref] [PubMed]