
New solutions to old problems—metabolic acidosis in chronic kidney disease
Introduction
Normal kidney function is essential to maintaining acid-base balance within the human body milieu. Renal dysfunction can result in the development of metabolic acidosis and subsequent pathophysiological changes in multiple organ systems. Current clinical practice guidelines set by the Kidney Disease Improving Global Outcomes (KDIGO) in 2013 suggested correction of metabolic acidosis in the setting of chronic kidney disease (CKD) to achieve normalization of serum bicarbonate, which should be 23–25 mEq/L, ideally 24 mEq/L (1). Provision of alkali therapy, namely sodium bicarbonate and citrate containing solutions have long been used as the defense against metabolic acidosis for decades. Recently, veverimer, a novel proton-binding polymer, has been proposed to provide an alternative mechanism for the correction of metabolic acidosis. In this editorial, we explore the pathophysiology for the development of metabolic acidosis in CKD and review therapies used to treat acidosis.
Pathophysiology—acidosis and the human body
Nonvolatile acids are naturally produced in the human body as a byproduct of metabolic activity, usually 1 mEq of proton per kg ideal body weight per day, particularly as a result of animal protein ingestion (2). Excretion of this acidic waste is of vital importance to prevent the disturbance of homeostasis that maintains processes integral to supporting life. Through ammoniagenesis and excretion of protons, the renal tubules efficiently clear these nonvolatile acids from the body. As tubular dysfunction and destruction worsen over time with progression of CKD, acid excretion diminishes and results in the retention of protons in the body leading to metabolic acidosis. The bicarbonate pool located in the extracellular fluid, which is refurbished daily by the kidneys, is typically adequate to buffer the daily acid load from both dietary intake and endogenous metabolic activity. However, renal dysfunction can lead to the inability to regenerate bicarbonate, potentially compounding to and worsening the metabolic acidosis.
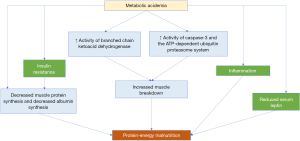
This acidosis can have deleterious effects including impairment in myocardial contractility, increased protein catabolism, systemic inflammation, insulin resistance, bone mineralization consequences, protein malnutrition, muscle wasting, and even mortality (2-5) (Figure 1). With respect to its effect on CKD, low serum bicarbonate levels in metabolic acidosis have been associated with a higher risk of progression of CKD to end-stage renal disease (ESRD) requiring dialysis (6,7). Even in patients with ESRD where correction of acidosis through dialysis is necessary, the presence of persistent metabolic acidosis continues to be associated with poor outcomes (8).
Current treatment strategies
Our current armamentarium against metabolic acidosis includes avoiding animal protein with higher proportion of plant-based diets and ingesting sodium bicarbonate and citrate formulations (sodium citrate and calcium citrate), which are rapidly metabolized to bicarbonate in the liver.
Dietary strategies
Investigations by Goraya and colleagues have assessed the effects of dietary changes on metabolic acidosis, especially a diet high in fruits and vegetables which has been shown to be less acidogenic (9,10). In a study assessing CKD stage 3 patients, a higher fruit and vegetable dietary content to target a reduction in dietary acid by 50% was enough to produce similar serum bicarbonate results as provision of sodium bicarbonate over the course of 3 years (10). Similar findings over one year were noted when assessing CKD stage 4 patients who were randomized to bicarbonate supplementation vs. a higher fruit and vegetable content diet (9). Interestingly, among CKD stage 3 patients, dietary acid reduction was also associated with a reduction in angiotensin II activity and preservation of GFR (10). A reduction in angiotensin II activity may curtail tubulointerstitial fibrosis, consequently slowing down progression of CKD (11). In addition to changes in acid-base balance, plant-based diets been shown to improve hypertension, decrease weight gain, reduce hyperphosphatemia, and decrease hyperfiltration, all of which are beneficial in the overall management of chronic kidney disease and its complications (12).
Medication strategies
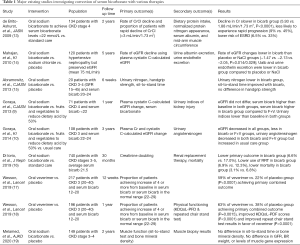
Other than diet, alkali therapy remains the mainstay treatment for metabolic acidosis. We reviewed and summarized the recent interventional studies using different alkali treatment options and their outcomes in Table 1 (9,10,13-20). Over the last several decades, many observational and small interventional studies have shown the benefit of alkali therapy. Many patients who are treated with sodium bicarbonate had a lower risk of requiring renal replacement therapy, and a lower risk of doubling of serum creatinine compared to patients who did not receive any treatment. Additionally, given that metabolic acidosis affects muscle metabolism through protein-energy malnutrition and inflammation (21), recent studies also noted the benefit of alkali therapy on muscle and bone changes. For example, in 2009, a study of 134 CKD stages 4–5 patients in the United Kingdom showed improvement in muscle mass among patients treated with sodium bicarbonate vs. placebo (13). However, Melamed and colleagues performed a similar trial of 149 patients with CKD stages 3–4 in the United States, but the authors did not find any significant difference in bone mineral density or muscle function among patients receiving sodium bicarbonate vs. placebo (19). Larger interventional studies are undoubtedly needed to confirm such findings of bicarbonate supplementation on physical function and the long-term effects on kidney function. In the absence of large trials to definitively demonstrate the benefit of alkali therapy, current international guidelines based upon moderate level evidence (2B) suggest treatment of metabolic acidosis with alkali therapy to achieve normalization of serum bicarbonate levels (1).
Full table
Controversially, the use of sodium bicarbonate has been thought to result in sodium loading, fluid retention and worsening hypertension, which might be especially detrimental among patients with CKD (15). However, in the trials comparing sodium bicarbonate to no treatment or placebo, there was no significant increase in blood pressure or weight gain, suggesting that sodium and fluid retention that is typically seen with sodium chloride therapy may be unfounded when using sodium bicarbonate (15,16,19). Nevertheless, some clinicians and patients anecdotally report worsening hypertension, volume overload, or difficulty tolerating a higher dose of sodium bicarbonate therapy. An additional restriction for oral therapy of acidosis pertains to citrate formulations in that it requires adequate liver function, and the dose response in patients with decreased hepatic function may be unpredictable. Thus, newer agents with better safety and efficacy profiles may expand our treatment options.
Veverimer, a novel agent against metabolic acidosis
The latest medication that has been recently developed specifically for the treatment of metabolic acidosis is veverimer. In a phase 3 trial published in the journal Lancet in 2019, veverimer demonstrated a promising novel treatment avenue for metabolic acidosis (17,18). Veverimer, a hydrochloric acid binder, is a non-absorbed polymer composed of low-swelling, spherical beads that selectively bind and remove hydrochloric acid from the gastrointestinal lumen through the feces. This new agent’s mechanism of action can be likened to the development of metabolic alkalosis with gastric lavage or emesis.
In a 12-week trial with a 40-week extension period over 29 sites in the United States and Eastern Europe, 217 patients with stage 3–4 chronic kidney disease (GFR 20–40 mL/min/1.73 m2) and metabolic acidosis (serum bicarbonate concentration of 12–20 mmol/L) were randomized to receive veverimer or placebo (17,18). This trial was primarily aimed to assess the safety profile of veverimer. At the end of the 12-week initial study period, more patients treated with veverimer achieved normalization of serum bicarbonate levels or an increase of 4 mEq/L or more in their serum bicarbonate levels compared to placebo (59% vs. 22%, P<0.0001). These results were similar at the end of the 52-week total extension trial (63% vs. 38%, P=0.0015), demonstrating the efficacy of veverimer.
As a secondary endpoint, the study investigators also assessed whether veverimer had any effects on quality of life and physical functioning, especially given evidence linking metabolic acidosis with protein catabolism and muscle wasting (22,23). Patients randomized to veverimer had improved physical function as determined by an increase in Kidney Disease Quality of Life-Physical Function Domain (KDQoL-PFD) score compared to placebo (12.1 points difference, SE 3.3, P<0.001) as well as improvement in repeated chair stand tests results (change of 4.3 vs. 1.4 s, P<0.001). The differences were consistent in subgroup analyses by age and sex. Overall though, these physical functioning results lend further evidence that correction of metabolic acidosis can improve the impairment that acidosis causes on muscle metabolism. Unfortunately, inflammatory markers such as interleukin-6 (IL-6) or C-reactive protein (CRP) were not assessed during this trial and inflammation remains an unmeasured confounder.
Serious adverse events occurred in 2% of veverimer-treated patients compared to 5% of placebo-treated patients, of which none were deemed to be related to the study drug. Headache was the only side effect with a significant between-group frequency difference but did not appear to be related to the study medication (15% of veverimer-treated vs. 25% of placebo-treated patients). Mild gastrointestinal (GI) side effects were also common (occurring in 21% of veverimer-treated patients vs. 26% of placebo-treated patients), as expected with polymer-binder drugs. Diarrhea and flatulence were the most common specific GI effects, occurring in approximately 6–7% of patients (no difference between the veverimer or placebo). There was also a notable proportion of patients with hyperkalemia in both groups (10–14%), though it is unclear if hyperkalemia is a related side-effect to the medication or as a result of pre-existing CKD.
Veverimer trial limitations and discussion
While the results of the veverimer trial appear promising, it is unclear if these results presented are the result of acidosis correction itself or if the medication has some other effects on physical functioning. A randomized trial between veverimer and sodium bicarbonate is warranted to ascertain if the results are driven solely by the correction of acidosis or by other mechanisms. Another limitation of the trial is the lack of information on the effects veverimer has on other medications. Veverimer’s effect on the acidic stomach environment is unclear, and may have an impact on the metabolism and absorption of many other medications that CKD patients take. Similarly, the common use of proton-pump inhibitors or H2-blockers for gastro-esophageal reflux disease by CKD patients may diminish the effect of veverimer by decreasing stomach acid secretion. In addition, while the trial stipulated separation of oral concomitant drugs and the study drug by at least 4 hours, this may not be practical in non-trial situations, especially with other commonly used medications such as phosphorous binders that are taken with meals. An additional limitation that arose with the trial was possible un-blinding due to the difference in appearance between veverimer and placebo. Other limitations to the trial include its mostly Caucasian patient population, excellent hypertension control and low degree of proteinuria, which may not reflect many patients with CKD. These are important questions that should be answered before wide-spread approval and use of veverimer.
While the veverimer trial was not sufficiently powered to assess progression of kidney disease or mortality, the results showed an impressive improvement in a combined outcome of mortality, use of renal replacement therapy, or a decline in eGFR of at least 50% (annualized IRR 4% vs. 12% in placebo, P=0.0224) (18). The difference in the combined outcome appears to be driven by higher death [4 (4%) in placebo vs. 0] and a higher increase in cases with confirmed decline in GFR of at least 50% [7 (8%) in placebo vs. 5 (4%)]. While the event rates are very low, the results appear promising. An interventional study with longer follow up time or a larger cohort should confirm these significant differences (18). As with studies assessing the effects of sodium bicarbonate therapy on metabolic acidosis, it would not be surprising if veverimer is also found to have similar beneficial effects with longer term studies.
Conclusions
We are enthusiastic about veverimer as a new development in the treatment of metabolic acidosis, an area which we are still using decades old therapies with a potentially unfavorable safety profile. Further studies comparing veverimer to the current standard of care including both medication and dietary interventions are needed to ascertain if the effects extend beyond those expected for the degree of acidosis correction. Also, with any new medication, it will be important to assess the cost effectiveness of veverimer compared to our existing and relatively inexpensive armamentarium against metabolic acidosis. We look forward with great anticipation to these future studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Cheng Yuan (Zhongnan Hospital, Wuhan University, Wuhan, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-2020-70). KKZ reports personal fees from Abbott, personal fees from Abbvie, personal fees from Alexion, personal fees from Amgen, personal fees from Astra-Zeneca, personal fees from Aveo, personal fees from Chugai, other from DaVita, personal fees from Fresenius Medical Services, personal fees from Genentech, personal fees from Haymarket, personal fees from Hospira, personal fees from Kabi, personal fees from Keryx, personal fees from Navartis, personal fees from Pfizer, personal fees from Relypsa, personal fees from Resverlogix, personal fees from Sandoz, personal fees from Sanofi, personal fees from Shire, personal fees from Vifor, personal fees from ZS-Pharma, personal fees from UpToDate, grants and personal fees from National Institutes of Health, personal fees from Baxter, personal fees from Dr Schaer, personal fees from PCORI, personal fees from Amag Pharma, outside the submitted work; The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:136-50.
- Kalantar-Zadeh K, Uppot RN, Lewandrowski KB. Case 23-2013: A 54-Year-Old Woman with Abdominal Pain, Vomiting, and Confusion. N Engl J Med 2013;369:374-82. [Crossref] [PubMed]
- Kraut JA, Madias NE. Adverse Effects of the Metabolic Acidosis of Chronic Kidney Disease. Adv Chronic Kidney Dis 2017;24:289-97. [Crossref] [PubMed]
- Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 2009;24:1232-7. [Crossref] [PubMed]
- Kopple JD, Kalantar-Zadeh K, Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl 2005;67:S21-7. [Crossref] [PubMed]
- Dobre M, Yang W, Chen J, et al. Association of Serum Bicarbonate With Risk of Renal and Cardiovascular Outcomes in CKD: A Report From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2013;62:670-8. [Crossref] [PubMed]
- Shah SN, Abramowitz M, Hostetter TH, et al. Serum Bicarbonate Levels and the Progression of Kidney Disease: A Cohort Study. Am J Kidney Dis 2009;54:270-7. [Crossref] [PubMed]
- Chen JLT, Kalantar-Zadeh K. Is an Increased Serum Bicarbonate Concentration during Hemodialysis Associated with an Increased Risk of Death? Semin Dial 2014;27:259-62. [Crossref] [PubMed]
- Goraya N, Simoni J, Jo CH, et al. A Comparison of Treating Metabolic Acidosis in CKD Stage 4 Hypertensive Kidney Disease with Fruits and Vegetables or Sodium Bicarbonate. Clin J Am Soc Nephrol 2013;8:371-81. [PubMed]
- Goraya N, Simoni J, Jo CH, et al. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 2014;86:1031-8. [PubMed]
- Remuzzi G, Perico N, Macia M, et al. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl 2005;68:S57-S65. [Crossref] [PubMed]
- Joshi S, Hashmi S, Shah S, et al. Plant-based diets for prevention and management of chronic kidney disease. Curr Opin Nephrol Hypertens 2020;29:16-21. [Crossref] [PubMed]
- de Brito-Ashurst I, Varagunam M, Raftery MJ, et al. Bicarbonate Supplementation Slows Progression of CKD and Improves Nutritional Status. J Am Soc Nephrol 2009;20:2075-84. [Crossref] [PubMed]
- Mahajan A, Simoni J, Sheather SJ, et al. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 2010;78:303-9. [Crossref] [PubMed]
- Abramowitz MK, Melamed ML, Bauer C, et al. Effects of Oral Sodium Bicarbonate in Patients with CKD. Clin J Am Soc Nephrol 2013;8:714-20. [Crossref] [PubMed]
- The UBI Study Group, Di Iorio BR, Bellasi A, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol 2019;32:989-1001. [Crossref] [PubMed]
- Wesson DE, Mathur V, Tangri N, et al. Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicentre, randomised, double-blind, controlled, phase 3 trial. Lancet 2019;393:1417-27. [Crossref] [PubMed]
- Wesson DE, Mathur V, Tangri N, et al. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet 2019;394:396-406. [Crossref] [PubMed]
- Melamed ML, Horwitz EJ, Dobre MA, et al. Effects of Sodium Bicarbonate in CKD Stages 3 and 4: A Randomized, Placebo-Controlled, Multicenter Clinical Trial. Am J Kidney Dis 2020;75:225-34. [PubMed]
- Kovesdy CP, Kalantar-Zadeh K. Oral bicarbonate: renoprotective in CKD? Nat Rev Nephrol 2010;6:15-7. [Crossref] [PubMed]
- Kalantar-Zadeh K, Mehrotra R, Fouque D, et al. Metabolic Acidosis and Malnutrition-Inflammation Complex Syndrome in Chronic Renal Failure. Semin Dial 2004;17:455-65. [Crossref] [PubMed]
- Mitch WE, Medina R, Grieber S, et al. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J Clin Invest 1994;93:2127-33. [Crossref] [PubMed]
- Song S, Chertow GM. Treatment of metabolic acidosis with an intestinal binder. Lancet 2019;393:1387-8. [Crossref] [PubMed]


