Lens regeneration in humans: using regenerative potential for tissue repairing
Introduction
Life-long growth and regenerative potential are the characteristics of the crystalline lens in humans. These characteristics derive from the embryonic origin of the lens, the epidermis ectoderm, which is known for the ability to regenerate after injury (1). In mammals, including humans, lens epithelial cells (LECs) can proliferate and differentiate to achieve certain extent of lens regeneration. Therefore, lens regeneration is a potential approach for visual function reconstruction after cataract surgery (2). In the process of lens regeneration, the differentiation of LECs is regulated by a variety of molecular signals and their interactions. Manipulating the molecular environment can accelerate and improve the optical quality of lens regeneration. This review summarizes the process of lens regeneration and its molecular mechanisms, and analyzes the strategies of clinical application of lens regeneration.
Structure and function of human lens
The crystalline lens is an epithelial-differentiated biconvex transparent organ. It consists of the lens capsule, the LECs, the lens fibers and the zonules, supported anteriorly by the iris and posteriorly by the vitreous body.
Refraction and accommodation are the most important physiological functions of the lens. Transparency of the lens allows transmission of light with wavelength up to 1,200 nm and is the prerequisite of refraction (3). In the Gullstrand model eyes, the cortex and nucleus of the lens are defined as a uniform refractive index, which is 1.386 and 1.406 respectively. In fact, the lens has a complex gradient refractive index (GRIN) (4). It has been confirmed by measuring the sagittal refractive index distribution and age change of the lens in vivo using magnetic resonance imaging (MRI) (5). The elastic biconvex morphology of the lens allows the eye to focus light from varying distances on macular, which is known as accommodation. Accommodation is accomplished by the lens and the ciliary body. When looking at distant objects, the ciliary muscles relax and the zonules keeps the tension of the lens capsule to make and the lens flatter, reducing the refraction power of the lens. On the contrary, the ciliary muscle contracts concentrically and the zonules relax to increase the refraction power of the lens to achieve near vision (6).
Development, growth and regeneration of human lens
Embryonic origin and development of the lens
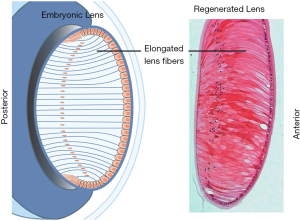
Lens development can be divided into two stages: the formation of lens vesicle and the formation of lens fibers (7). In embryonic stage, after the optic vesicle contacts with the epidermis ectoderm, the thickening of the epidermis ectoderm is induced to form the lens plate, which is the primordium of the lens. The lens plate invaginates into the optic cup and gradually separates from the epidermal ectoderm, forming lens vesicles. At the beginning, the lens vesicle was composed of a single layer of epithelium. The cells in the anterior wall of the vesicle were cuboidal and differentiated into lens epithelium, while the cells in the posterior wall were high columnar and elongated towards the anterior wall gradually to form the primary lens fibers. The intra-vesicle space gradually shrinks, and the lens becomes a solid structure. Since then, the epithelial cells in the equatorial region of the lens have been proliferating, growing and forming secondary lens fibers.
Growth of the lens after birth
The fibers distributed in the lens equator contain more cellular organelles, and the activity of protein synthesis is higher. Mature fibers lack active physiological activity. When the elongation of lens fiber is completed, the end of lens fiber is separated from the lens epithelium or capsule, and then docked with the lens fiber on the opposite side to form a lens suture. Lens suture plays an important role in accommodation. Compared with other species, such as birds, human lens with discontinuous suture has more powerful accommodation ability (8). Throughout the entire lifetime, newly differentiated lens fibers are constantly superimposed on the inner lens fibers to form an onion-like concentric layered structure. This process slows down with age (9).
The epithelial cells in the lens equator continue to divide and grow throughout the entire lifetime, so the weight and volume of the lens are constantly changing.
Regeneration of the lens after injury
Lens regeneration in lower vertebrates
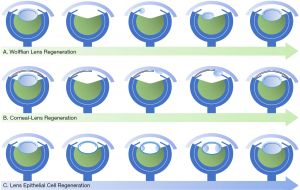
Both lower and higher vertebrates have the ability of lens regeneration, however, the mechanisms and manifestations of which are different (Figure 1). After the whole crystalline lens is removed, the lower vertebrates achieve lens regeneration originated from other types of neighboring cells, which can be further divided into the Wolffian lens regeneration and the corneal lens regeneration according to the cell origins (Figure 1A,B) (10,11). The representative model of Wolffian lens regeneration is the newts, which are capable of lens regeneration throughout life. After the crystalline lens is removed, the pigmented epithelial cells (PECs) on the dorsal side of the iris firstly dedifferentiate, and then differentiate into LECs, regenerating a new intact lens. The representative model of corneal lens regeneration is the Xenopus. In its early life stages, corneal-derived cells (stem cells or transient amplify cells presented in the corneal stroma) differentiate to LECs and regenerate a new lens after the whole crystalline lens is removed. The prerequisite of lens regeneration in lower vertebrates is that the original crystalline lens is completely removed, triggering retina-originated regeneration signals to act on specific tissue cells (1). In contrast to lower vertebrates, lens regeneration in higher vertebrates can only achieved when the lens capsule and cells are preserved. More details will be discussed in the following paragraphs. Although the regeneration process is different among species, the strong capability of lens regeneration in lower vertebrates helps us to profoundly understand regulatory mechanisms of lens regeneration for further clinical translation and application.
Lens regeneration in mammals
In mammals, lens vesicles are formed by lens primordium invagination during embryonic development, and lens primordium invagination is caused by signals from the retinal primordia (12). Subsequently, the cells located in the posterior capsule of the lens elongate to reach the anterior capsule and fill the space in the lens vesicle. After birth, LECs only present in the anterior capsule of the lens. The epithelial cells located in the lens equator continue to differentiate into lens fiber cells and elongate to the anterior and posterior direction of the lens equator.
As early as the 19th century, researchers have found that mammalian lens regeneration is different from lower vertebrates. After the lens capsule is removed, no signs of lens regeneration can be observed (13). However, if only the contents of the lens are removed and the epithelial cells under the lens capsule and the anterior capsule are preserved, the lens can reproduce the process similar to that of embryonic development and form a regenerated lens (14,15). We removed the lens contents in New Zealand albino rabbits. On the first day after operation, the lens anterior capsule attached to the posterior capsule. On the fourth day after operation, the posterior capsule of the lens was covered with migrated LECs, and eosinophilic substance could be seen between the anterior and posterior capsule of the lens. On the seventh day after operation, the LECs on the posterior capsule elongated and contacted with the LECs under the anterior capsule, and the eosinophilic substance between the anterior and posterior capsule disappeared. This process reproduces the formation of primary lens fibers during embryonic development (Figure 2; unpublished data). However, Liu et al. found that compared with a natural lens, the epithelial cells of the regenerated lenses had some morphological changes, including overly dense, indented nuclei, some edematous mitochondria, and an expanding endoplasmic reticulum (16).
Protein composition of mammalian regenerated lens
The development of new technology has brought new enlightenment to the study of the mechanism of lens regeneration. Previous study on the regeneration of newt lens at the protein level revealed the important biological processes of programmed regeneration, such as inflammation is beneficial to host defense and cell activation, and tissue homeostasis can be achieved by regulating ROS and DNA repair (17). Different from other organisms, the lens regeneration ability of newt does not decrease with age. Sousounis et al. found that the robustness of the old newt regenerated lens was similar to that of the young newt lens at the transcriptome level after 19 times of lens regeneration (18). In contrast to lower vertebrates, Liu et al. found through proteomic analysis that the expression of crystallin in the regenerated lens of rabbits of different ages was similar to that of the lens of adult rabbits, but different from that of young rabbits (2 weeks old) (16). These results show that the proteome of mammalian regenerative lens mimics a “mature” lens, from this point of view, the regeneration process does not fully simulate the embryonic development. Congenital cataracts caused by gene defect is one of the main causes of lens opacification in infants (19-22). The proteome of mammalian regenerative lens indicates that in the process of lens regeneration after surgery, even without additional molecular intervention, transparent regenerated lens may be obtained for congenital cataracts caused by abnormal embryonic crystallin (16). Wu et al. constructed the proteomic database of human regenerated lens and performed whole genome sequencing of congenital cataract (23), providing the basis for further understanding the protein composition of human regenerative lens.
Limitations of current surgical treatment for infantile cataracts
Surgery is the common treatment for cataract. Ophthalmologists found some transparent tissue growing in the lens capsule after cataract surgery in humans (24-26). This phenomenon of postoperative proliferation suggests that the human lens has the ability of regeneration. However, in the conventional cataract surgery, a 6 mm anterior capsule opening is made, which results in a large wound and loss of a great number of LECs and lens epithelial stem cells. After the surgery, the residual LECs in the capsule proliferate disorderly to form locally pearl-like structure, or go through epithelial-mesenchymal transition (EMT), and subsequently become opaque structure, which is known as posterior capsular opacification (PCO) (27).
Intraocular lens (IOL) implantation is the most commonly used way for refractive correction after lens removal. However, the application of IOL implantation in children under 2 years old has many limitations. The eyeball of infant is still developing leading to unpredictable myopia shift after surgery (28,29). The disordered growth of residual lens cells in children after cataract extraction may cause visual axis opacification (VAO) and even secondary blindness due to inflammation and proliferation. In addition, IOL dislocation, poor biocompatibility, and loss of accommodation ability all seriously affect the visual prognosis in young children. It is reported that early IOL implantation in infants significantly increased the risk of reoperation (30). Therefore, the use of IOL implantation in children under two years old remains controversial.
In order to improve the prognosis of cataract surgery in infants, we have established a novel surgical technique to promote lens regeneration and reduce risk of postoperative complications. It will be described in detail below.
Using endogenous stem cells to achieve lens regeneration in humans
Microenvironment of lens regeneration
Age and species
Similar to Xenopus, the lens regeneration ability of mammals is greatly affected by age (31). The younger the age is, the faster the regeneration speed is. After lens content removal in 8-week-old New Zealand albino rabbits, the regenerated lens fibers had the same morphology as normal lens fibers; 14 days after operation, the fibers were neatly aligned in the cross section of the regenerated lens and showed a hexagonal structure (Figure 3; unpublished data).

In 2–4-month-old cynomolgus monkeys, we also observed in situ lens regeneration after minimally invasive lens aspiration, and the regenerated lens fibers gradually grew from the periphery of the lens (a doughnut-like structure) to the center. The biconvex morphologic feature of the crystalline lens was observed three months after operation. Compared with New Zealand albino rabbits, the regeneration speed of cynomolgus monkey lens is slow, and the regenerated lens can reach 50% of the normal lens thickness 5 months after operation (32).
Lens regeneration can also be observed in young children (32). The process of regeneration is similar to that of New Zealand albino rabbits and cynomolgus monkeys.
LECs and residual lens materials
In mammals, the LECs cover the anterior capsule and equator of the lens after birth. Different from lens fibers, LECs retain a complete set of nuclei and organelles and play an important role in maintaining the homeostasis and metabolism of the lens. Among them, some cells maintain stem cell characteristics. Lin et al. found that Bmi-1 and Pax-6 are crucial factors to maintain the characteristics of lens stem cells through linear-tracing experiments (32). The lens stem cells or precursor cells in the equator gradually elongate and differentiate into lens fiber cells during their migration to the arc region, which is the basis of the life-long growth of the lens. After the lens content is removed, a large number of lens stem cells are activated, and a transient period of rapid regeneration of lens fibers occurs.
In the process of mammalian lens regeneration, LECs under the anterior capsule of the lens play a role in guiding the docking and alignment of lens fibers. When the LECs are injured, the surrounding LECs move to the defect area and EMT occurs, which leads to scarring and opacity under the anterior capsule (Figure 4; material from: Lin et al., “Lens regeneration using endogenous stem cells with gain of visual function”, Nature 2016, Nature Publishing Group) (32). At the same time, the process of lens fiber elongation and docking to this area is disturbed, resulting in opacity of the regenerated lens (33,34). Excepting the LECs, and lens stem cells under the anterior capsule and at the equator of the lens, other residual lens materials or exogenous injected materials will affect the differentiation and orderly alignment of the regenerated lens fibers, resulting in opacity of the regenerated lens (35).
Capsulorhexis size and integrity of the lens capsule
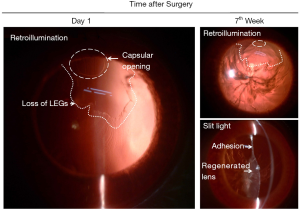
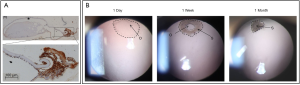
The shape of the regenerated lens depends on the integrity and closure of the lens capsule, and the adhesion between the lens anterior and posterior capsules (36,37). Previous studies showed that there were different amounts of fibrin at the incision of lens capsule, which could seal the capsule with scar. Gwon et al. inserted a collagen patch to restore the closure of lens capsule and filled the lens capsule with air, Healon or perfluoropropane gas to prevent adhesion and wrinkling, so as to improve the shape of the regenerated lens. As a result, the crystalline lens regenerated faster and completely filled the capsule at 5 weeks, which was spherical and had normal cortical structure, but with nuclear opacity. In the Healon or perfluoropropane group, there were adhesion of lens capsule, increase of scar in anterior capsule or delayed regeneration (35). Lin et al. observed the contraction of micro-capsulorhexis, the formation of local opacification and the re-closure of the capsule after lens surgery in primates and human infants (Figure 5; unpublished data). Histologically, it was EMT of LECs, which posed a dilemma leading to either lens regeneration or after cataract (38).
Minimally invasive lens-content removal surgery (MILS)
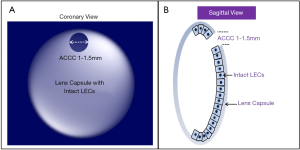
Integrity of lens capsule, LECs and stem cells are prerequisites for functional in situ regeneration of mammalian lens. The progress of surgery technology, including the emergence of femtosecond assisted cataract surgery (FLACS), has improved the success rate of micro capsulorhexis. We have established a novel surgical strategy, MILS (Figure 6) (32). The 1–1.5 mm capsulorhexis opening located in the periphery of the anterior capsule of the lens is beneficial to the contraction and closure of the capsulorhexis opening in the early postoperative period, and to reduce the influence of the scar at capsule opening on the morphology of the regenerated lens. It may be beneficial to further improve the shape of the regenerated lens by using implants instead of relying on EMT to achieve capsular closure, and by preventing the adhesion of the anterior and posterior capsule at capsular opening.
The LECs and lens stem cells under the anterior capsule and at the equator should be protected during the process of lens content removal. The operation of hydrodissection should be as gentle as possible. In the process of hydrodissection, viscoelastic agent can be used instead of balanced salt solution (BSS) to reduce the risk of detachment of anterior subcapsular cells. The lens nucleus of infant mammalian and human congenital cataract children is soft but sticky. Sufficient hydrodissection helps to avoid inserting phacoemulsification handpiece and irrigation and aspiration handpiece into the capsule and protect the integrity of the anterior subcapsular cell layer (see Table 1).
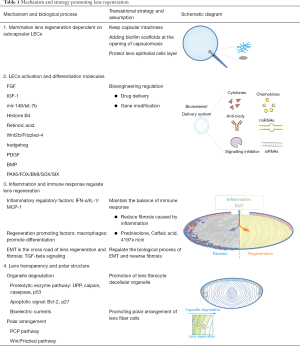
Full table
Optical properties of the regenerated lens
The optical quality of regenerated lens, including transparency, refractive power, aberration, etc., defines whether it is functional regeneration. Most studies attempt to measure or qualitatively evaluate the refractive power of the regenerated lens, but the local opacity and structural abnormality of the regenerated lens affect the accuracy of the measurement. Some studies give qualitative statements. In the eyes of the rabbits that did not receive the implants, Gwon showed a picture of the fundus clearly visible (39).
Lin et al. evaluated the refractive power of the regenerated lens in New Zealand albino rabbits after MILS, and found that the average growth rate was 15.6 D at 15th month after operation, which was similar to that of the normal lens; for cynomolgus monkeys, a biconvex lens was formed 5 months after operation, and the visual axis was transparent; in the fundus examination 7 weeks after MILS, the retina was clearly visible, suggesting that the regenerated lens is transparent (32).
After MILS in infants, the function of the regenerated lens was evaluated. It was found that the refractive power of the lens increased significantly from 1 week to 8 months after the operation. At the same time, the average accommodation response of the regenerated lens increased to 2.5 D, which was much higher than that of the control group (P<0.001) (32).
Molecular mechanism and strategy promoting lens regeneration
Activation and differentiation of lens stem cells
In the process of lens regeneration in amphibians such as newt, the dorsal iris pigment epithelial cells (PECs) dedifferentiation, depigmentation, proliferation and then gradually differentiated into lens fiber cells after the formation of a lens vesicle. However, humans and mammals can only regenerate the lens cells originated from the lens epithelial (stem) cells remaining in the lens capsule, rather than transdifferentiate from other eye tissues.
Epigenetics
The process of transdifferentiation is also regulated by epigenetics. The dorsal iris PECs have the potential to dedifferentiate and recover into stem cell-like cells, while the ventral PECs do not. Study of histone modification found that there was a general activation state in both iris during dedifferentiation, while the inhibitory marker H3K27me3 was uniquely retained in the front iris at this stage (40,41). Another study has found that an oocyte-type connexin histone B4 is a key factor in the process of lens transdifferentiation. The expression of key genes in lens differentiation of newt with B4 gene knockout is significantly changed, and almost no γ-crystallin is expressed (42).
Chromatin change is a new characteristic of lens differentiation (43). The change of chromatin accessibility can regulate the expression of multiple genes through competitive physical DNA binding. The organization of accessible chromatin across the genome reflects a network of permissible physical interactions (44).
Zhao et al., used ATAC-seq technology to detect the accessibility of lens chromatin, analyzed the initiating and terminating dynamics of chromatin and the change of mRNA in the process of mammalian lens differentiation, as well as the potential role of candidate enhancers in time and space gene regulation during lens development was analyzed (45).
Growth factors
Many growth factors may activate the residual LECs to re-enter the cell cycle, and then proliferate and differentiate to a regenerated lens. Coagulation fibrin formed after newt injury can recruit macrophages to secrete fibroblast growth factors (FGFs), and then initiate the process of dedifferentiation and the cell cycle reentry (46,47). FGFs and its receptors are expressed during lens regeneration, and treatment with the exogenous FGFs can induce the formation of a new lens in vitro (48-51). Inhibition of FGF signaling pathways alone can eliminate lens regeneration (52). The neuroretina is considered to be the origin of cytokines, and the separation of the neuroretina from the iris will prevent lens regeneration (53,54), moreover neuroretina-derived FGF factors are crucial for lens fiber development in vivo (55). FGF signaling is required for lens regeneration in Xenopus laevis, which could initiate transdifferentiation of cornea epithelial cells in culture (51). In addition, FGF appears to be important during lens development including lens epithelial cell proliferation, fiber differentiation (56-59) and fiber cell elongation (60).
Recently, it has been found that lens differentiation is controlled by the balance of platelet-derived growth factor (PDGF) and FGF signals, and PDGF/PI3K and FGF/MAPK signal pathways antagonize each other. The maintenance and differentiation of lens progenitor cells can be balanced by selective activation (61).
Transcription factors
Transcription factors closely related to lens development also play a key role in the process of regeneration. Pax-6 (the main regulator of lens development) is involved in different processes from lens basal plate to lens fiber differentiation (62). Pax-6 is re-expressed in newt during dedifferentiation of back iris PECs and subsequent lens regeneration (63). Overexpression of six-3 combined with retinoic acid treatment or inhibition of pax-6/six-3 upstream molecule BMP can achieve ventral lens regeneration of newt (64). Lin et al. found that pax-6 and BMI-1 are the key points that determine the self-renewal of lens stem cells through a series of experiments such as stem cell lineage tracing and tissue-specific gene knockout (32). By knocking down expression of Pax-6 in newt, Madhavan et al. found that lens regeneration was significantly retarded, however, induction of dedifferentiation was not inhibited. It suggested that Pax-6 regulates proliferation but not differentiation at later stages of regeneration (63). Sox2 expression is dependent on Pax6, these two transcription factors have been implicated in early events in lens induction and have been proposed to cooperate functionally by forming a co-DNA-binding partner complex (65) and moreover, Pax6-Sox2 inter-regulation is stage-dependent (66). Other important transcription factors related to lens development are Prox1 (59,67), Foxe3 (68-71), HSF4 (70,72,73), c-MAF (70,73,74), MIP (45), etc. In addition, DNase-seq, ACTA-seq and other new epigenetic technologies are conducive to the discovery of new transcription factors or DNA binding regions that regulate the lens differentiation, such as gatad1 and NF1 (45).
Other important molecular and signaling involved in lens regeneration include retinoic acid (75-78), Wnt2b/Frizzled-4 (79,80), hedgehog (81) and BMP/TGF beta (82) (see Table 1).
Using combinatorial growth factor media in a certain order and concentration, human stem cells could differentiate into lentoid bodies in vitro (83,84). By treatment of BMP4/BMP7, EGF/TGFα, HGF, IGF1/insulin and PDGF-AA into ROR1+ iPS-derived LECs, in a 3-stage protocol, Murphy et al. achieved light-focusing human micro-lenses (83). These in vitro cultured micro-lenses can simulate the lens development process, including lens fiber differentiation and capsule formation. Additionally, they were similar to the human lens in protein level and structural level.
The diameter of these micro-lenses is 80–200 um, while the human lens is about 10 mm. In fact, the micro-lens is no longer for transplantation therapy, but aiming at a promising cataract disease and drug screening model. This lens development system may be useful for understanding how multiple defined growth factors and signalling cascades that activate progressive that lead to establishment of a functional lens.
Roles of EMT and immune response during the process of lens regeneration
EMT is the common process of lens regeneration and fibrosis
After being activated into a new cell cycle, stem cells further proliferate and differentiate, in which the transformation of residual epithelium into mesenchymal cells, in another word, EMT, is a necessary process of repair after injury. The whole genome expression and cluster analysis confirmed that the known EMT regulatory factors were expressed in the early stage of regeneration, the transcripts related to injury and extracellular matrix remodeling were significantly increased, and the lens fiber differentiation process started (85). This process is similar to the fibrosis process after lens injury. EMT also occurs in the pathological process of scar formation and fibrosis after lens injury. It has been confirmed that TGF-beta is the main regulatory factor of lens fibrosis after lens injury. Therefore, many studies have shown that EMT is the crossroads of cell stemness and fibrosis (86,87), and the regulation of EMT process is expected to become a new direction of regenerative medicine.
The ability of lens regeneration is negatively correlated with the evolution of immune system
From the general trend of species evolution, the maturity of the immune system is inversely proportional to the ability of regenerating damaged tissue (88). The decrease of lens regeneration ability with age may also be negatively related to the development of ocular immune system. Adults have a well-developed special eye immune system to ensure that when damage occurs, it is not caused by the side effects of autoimmune response that lead to opacity of the refractive stroma (89). It is characterized by a blood-eye barrier composed of few lymphovascular tissues in structure and tight junctions between epithelial cells and vascular endothelial cells; Functionally, when the eyeball is injured or the antigen is injected into the eye through the anterior chamber, the timely removal of the mononuclear macrophages of F4/80+CD11b+ does not cause delayed type hypersensitivity and complement-mediated cellular immunity to protect the intraocular cells from membrane cleavage and other damage. Children have stronger lens regeneration ability but immature ocular immune system, and have stronger inflammatory and proliferative responses after cataract extraction than the elderly. The prevalence rate of secondary cataract after cataract surgery is 100% (90) in children and 40% in adults (91,92).
A balanced immune response is beneficial to lens regeneration
The regeneration and fibrosis of remained capsular after intracapsular lens extraction are the two outcomes of lens post-injury repairation. Studies have shown that the two different outcomes of tissue regeneration and scar formation are closely related to immune regulation (93,94). Inflammation and immune cell recruitment are protective signs of early injury. Immune cells not only help to remove fragments in the wound, but also secrete a large number of signal molecules to induce appropriate cell proliferation and differentiation procedures, which are essential for successful regeneration. Additionally, the removal of immune cells from animals will reverse tissue regeneration into scar formation (95,96). On the other hand, excessive polarization of pro-inflammatory immune cells or secretion of pro-inflammatory cytokines lead to the imbalance of pro-inflammatory immune regulation and pro-tissue regeneration immune regulation, thus inhibiting the process of regeneration leading to scar formation. Therefore, successful regeneration requires a balanced immune response, an appropriate number of accurately polarized immune cells and a well-regulated network of cytokines.
The lens has been considered to be an immune privileged organ, in which tissue injury failed to evoke a conventional immune response. Even though, the immune system still has a special way to regulate the regeneration of eye tissue. Unlike the wound healing of skin, heart or limbs in vivo, because the lens is a transparent non-vascular tissue, the repair process does not include the process of angiogenesis, and its immune cells are mainly recruited through the blood vessels in the iris and ciliary body. Recently, it has been found that the lymphatic-specific marker, lymphatic vessel endothelial hyaluronic acid receptor (LYVE-1) reaches the lens along the suspensory ligament connecting the lens and the ciliary body, which proves that the lens is connected with the lymphatic system, and providing a potential structure basis of the immune cycle (97).
A variety of cytokines and growth factors form a lens immune regulatory network with immune cells. The intraocular infiltration of neutrophils and macrophages was confirmed in the lens capsule after cataract extraction in mice, and the recruitment time was 18 hours and the third day after operation, respectively, which was later than the upregulation of cytokines (98). Due to the destruction and stimulation of the blood-aqueous humor barrier caused by surgery, many kinds of cytokines and growth factors are overexpressed in aqueous humor, such as interleukin-1 (IL-1), transforming growth factor β-s (TGF-βs), FGF-2, IL-6, epidermal growth factor (EGF) and hepatocyte growth factor (HGF). In the mouse cataract extraction model, it was found that the genes regulating the innate immune response were significantly up-regulated after operation in 24 hours, such as CXCL1, S100a9, CSF3 and COX-2 (98). The congenital cataract patients have higher regenerative ability than the age-related cataract patients, the concentrations of G-CSF, IFN-α2, IL-1 αand IL-7 in aqueous humor of congenital cataract group were higher than those of age-related cataract group. In addition, the EGF and IL-3 were positively correlated with age, while IL-8 and monocyte chemoattractant protein (MCP-1/CCL2) were negatively correlated with age (99).
All of the above proved that the immune inflammatory reaction is involved in the process of lens regeneration after injury. The cytokines and growth factors involved can be divided into pro-inflammatory type and pro-regeneration type according to their functions. After polarization, immune cells secrete specific types of cytokines and growth factors to participate in the regulation of regeneration. For instance, macrophages are the central regulatory cells for repair after injury. Macrophages pro-inflammatory M1 (IFN-γ) polarize to M2 (IL-4) and secrete the pro-regenerative cytokines, which can promote the regeneration of injured tissue (93). In the process of lens regeneration of adult salamander, macrophages participate in the phagocytosis of melanosomes of iris epithelial cells (100,101), and secrete regeneration promoting factor FGF to activate the transdifferentiation of iris cells (49). Higher levels of macrophage colony factor G-CSF and GM-CSF in children after cataract surgery suggest stronger macrophage mobilization after lens injury in infants (99), which may be related to the stronger regeneration ability of children. Proteomic analysis suggests that bone myeloid cells are also involved in the initiation of regeneration in newts. After lens removal, myeloperoxidase (MPO) gene products such as MPO are specifically located in the regeneration site of the dorsal iris, rather than the ventral iris (17). The activation of immune cells may also be regulated by other tissues in the eye. Lymphangiogenesis from cornea promotes the destruction of lens and subsequent lens regeneration, which can be accelerated by the transplantation of dendritic cells. The induction of regeneration depends on the spleen, and may depend on the mobilization of other immune cells after antigen presentation (102). By knocking down expression of lens-specific N-cadherin in mouse, a lens degeneration model was built and an immune response throughout the eye was observed, including cornea, vitreous humor, and retina; Also, resulting in immune cells populating the lens (97). In addition, complement 3 and complement 5 were expressed in newt regenerated limb and lens.
The immune system has two sides in regulating tissue repair and regeneration, so promoting adult tissue regeneration by regulating the immune system has become an attractive approach in regenerative medicine. The main strategies include initiating the healing process by releasing pro-inflammatory regulators, or promoting the decomposition phase by releasing anti-inflammatory regulators from anti-inflammatory/anti-fibrotic macrophages. More complex strategies rely on the sequential transmission of pro- and anti-inflammatory molecules to exert more comprehensive control over the tissue healing process.
At present, there is no report of directly promoting lens regeneration by delivering immunomodulatory factors. In the treatment of postoperative lens fibrosis, laser therapy is mainly used to open the turbid fibrosis capsule. There are also studies on the use of drugs to inhibit inflammation in the treatment of PCO such as prednisolone (103), caffeic acid (104), cellular immunotoxin 4197X-ricin (105), etc. At present, the limitations of drug therapy and other therapies are mainly to control the toxic reaction of corneal endothelial cells. Further research on the immune regulation of lens regeneration can bring us new sights on PCO treatment (see Table 1).
Molecular basis for maintenance of lens transparency
In addition to preventing lens capsule fibrosis, the transparency of regenerated lens needs to meet many conditions. In the differentiation of lens fiber cells, programmed removal of organelles helps to improve transparency (106). Apoptosis signal and proteolytic enzyme pathway are related to lens fiber cell differentiation and organelle loss (106), including Bcl-2 and apoptosis family inhibitors, tumor necrosis factor, p53 and its regulatory factors (such as Mdm2) and proteolytic enzymes (including caspases, cathepsins, cathepsins, p53, etc.), calpain and ubiquitin-proteasome pathway. The lens contains more protein than any other tissue. Unfolded and abnormal accumulation of the protein may interfere LECs denucleation and leading to cataract. Lyu et al. found that the accumulation of p27 prevented the phosphorylation of laminin A/C and lens fiber cell denucleation, which may be related to unfolded protein reaction (UPR) (33). The mechanism of chromatin regulation is also involved in the process of lens enucleation, for example, abnormal chromatin remodeling enzyme Snf2h leads to the failure of lens cell enucleation in mice (107). In recent years, Cao et al. have studied the bioelectric signals produced by Na/K pump current at the equator of mammalian lens and found that the enucleation of equator LECs can be activated via depolarization of hyperpolarized membrane potential difference (Vmem) (108).
The transparency of the lens also depends on the correct assembly of epithelial and fibrous cells into a functional three-dimensional structure. Dawes et al. found that there is a primary cilia on the hexagonal tip surface of each lens fiber cell, which polarizes toward the front pole, and the inactivation of genes encoding components of the Wnt/planar cell polarity (PCP) pathway, such as Rac1, Vangl2 and Celsr1, destroys the orientation of cilia and the morphology of lens fibers (109). Epithelial-derived Wnt guides the arrangement and orientation of lens fibers by triggering the PCP pathway and the shift of frizzled and centrosome to the top of elongated fiber cells (109,110) (see Table 1).
Conclusions
Regenerative medicine has brought light to the treatment of many refractory diseases. With the progress of surgical technology, biomaterials and experimental methods, the understanding of the essence, law and transformation strategy of regeneration has been deepened and advanced. The clinical translation of regenerative medicine to achieve functional rehabilitation is closer to reality than ever before. This review discussed lens regeneration from the perspective of clinical application. Examples of lens regeneration are common in biology and can be found in mammals and amphibians. However, the interesting question is that there are substantial differences in the process of lens regeneration in different species, specifically how similar the molecular mechanism used for lens regeneration is to that used in the initial embryonic development. Wolffian lens regeneration uses a unique mechanism different from embryonic development. The lens regenerates through the transdifferentiation of the iris. The developmental lineage of the iris is different from that of the cornea or lens. On the other hand, corneal lens regeneration seems to be more closely following the extensive cellular signal network applied to the surface ectoderm during the initial embryonic development of lens, because both cornea and lens originate from the head ectoderm covering the ocular cup. In mammals, the process of lens regeneration depending on lens epithelium is similar to that in embryonic development, but the composition of protein expressed is different, and the ability of lens regeneration is also the most limited. With the increase of age, lens regeneration gradually weakens and becomes disordered, and gradually loses the ability of functional regeneration. From the technical strategy, minimally invasive extraction of lens content makes it possible to obtain a complete lens capsule and maintain an intact anterior subcapsular cell layer. The use of biomaterials may help the early closure of the lens capsule and avoid the formation of scar between the anterior and posterior capsule. In terms of molecular regulation, according to the different stages of functional regeneration, the key problems to be solved in improving the quality of mammalian regenerated lens are to sequentially activate and deactivate stem cells, to balance regeneration and fibrosis, and to induce normal differentiation and orderly arrangement of lens fibers. The findings discussed in this review are of great significance for the wider clinical application of lens regeneration in the future. The therapeutic concept of MILS combined with microenvironment manipulation to activate endogenous stem cells for functional regeneration of organs in situ can also be extended to other tissues and organs with strong self-renewal and repair ability.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81873675, 81822010).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Andrzej Grzybowski) for the series “Recent developments in cataract surgery” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-2019-rcs-03). The series “Recent developments in cataract surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Henry JJ, Thomas AG, Hamilton PW, et al. Cell signaling pathways in vertebrate lens regeneration. Curr Top Microbiol Immunol 2013;367:75-98. [Crossref] [PubMed]
- Sukhija J, Kaur S. Nature nurtures: lens regeneration, a breakthrough in ophthalmology. Ann Eye Sci 2017;2:17. [Crossref]
- Hejtmancik JF, Shiels A. Overview of the Lens. Prog Mol Biol Transl Sci 2015;134:119-27. [Crossref] [PubMed]
- de Castro A, Birkenfeld J, Maceo B, et al. Influence of shape and gradient refractive index in the accommodative changes of spherical aberration in nonhuman primate crystalline lenses. Invest Ophthalmol Vis Sci 2013;54:6197-207. [Crossref] [PubMed]
- Pellegrino A, Burd HJ, Pinilla Cortes L, et al. Anterior lens capsule strains during simulated accommodation in porcine eyes. Exp Eye Res 2018;168:19-27. [Crossref] [PubMed]
- Kasthurirangan S, Markwell EL, Atchison DA, et al. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J Vis 2011;11:19. [Crossref] [PubMed]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol 2001;17:255-96. [Crossref] [PubMed]
- Kuszak JR, Zoltoski RK, Tiedemann CE. Development of lens sutures. Int J Dev Biol 2004;48:889-902. [Crossref] [PubMed]
- Zhang T, Liu Y, Wu M, et al. Compare the proteasome activity in the epithelium of human age-related cataract and normal lens. Yan Ke Xue Bao 2006;22:89-91, 102.
- Eguchi G, Eguchi Y, Nakamura K, et al. Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nat Commun 2011;2:384. [Crossref] [PubMed]
- Barbosa-Sabanero K, Hoffmann A, Judge C, et al. Lens and retina regeneration: new perspectives from model organisms. Biochem J 2012;447:321-34. [Crossref] [PubMed]
- Iribarren R. Crystalline lens and refractive development. Prog Retin Eye Res 2015;47:86-106. [Crossref] [PubMed]
- Gwon A. Lens regeneration in mammals: a review. Surv Ophthalmol 2006;51:51-62. [Crossref] [PubMed]
- Gwon A, Gruber LJ, Mantras C. Restoring lens capsule integrity enhances lens regeneration in New Zealand albino rabbits and cats. J Cataract Refract Surg 1993;19:735-46. [Crossref] [PubMed]
- Gwon A, Gruber L, Mantras C, et al. Lens regeneration in New Zealand albino rabbits after endocapsular cataract extraction. Invest Ophthalmol Vis Sci 1993;34:2124-9. [PubMed]
- Liu X, Zhang X, Liu Y, et al. To establish and observe the experimental lens regeneration model in rabbits. Yan Ke Xue Bao 2002;18:230-4, 248.
- Sousounis K, Bhavsar R, Looso M, et al. Molecular signatures that correlate with induction of lens regeneration in newts: lessons from proteomic analysis. Hum Genomics 2014;8:22. [Crossref] [PubMed]
- Sousounis K, Qi F, Yadav MC, et al. A robust transcriptional program in newts undergoing multiple events of lens regeneration throughout their lifespan. Elife 2015;4:e09594. [Crossref] [PubMed]
- Zhang W, Liu Z, Bao X, et al. CHIP Knockdown Reduced Heat Shock Response and Protein Quality Control Capacity in Lens Epithelial Cells. Curr Mol Med 2015;15:652-62. [Crossref] [PubMed]
- Wu M, Zhang X, Bian Q, et al. Oligomerization With Wt αA- And αB-crystallins Reduces Proteasome-Mediated Degradation of C-terminally Truncated αA-crystallin. Invest Ophthalmol Vis Sci 2012;53:2541-50. [Crossref] [PubMed]
- Liu Z, Taylor A, Liu Y, et al. Enhancement of ubiquitin conjugation activity reduces intracellular aggregation of V76D mutant γD-crystallin. Invest Ophthalmol Vis Sci 2012;53:6655-65. [Crossref] [PubMed]
- Liu Y, Zhang X, Luo L, et al. A novel alphaB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci 2006;47:1069-75. [Crossref] [PubMed]
- Wu X, Liu Z, Zhang X, et al. Proteomics analysis and proteogenomic characterization of different physiopathological human lenses. BMC Ophthalmol 2017;17:253. [Crossref] [PubMed]
- Luo L, Lin H, Chen W, et al. In-the-bag intraocular lens placement via secondary capsulorhexis with radiofrequency diathermy in pediatric aphakic eyes. PLoS One 2013;8:e62381. [Crossref] [PubMed]
- Mandal AK, Gollakota R. Soemmering's Ring. Ophthalmology 2017;124:1064. [Crossref] [PubMed]
- Bhattacharjee H, Deshmukh S. Soemmering's ring. Indian J Ophthalmol 2017;65:1489. [Crossref] [PubMed]
- Fișuș AD, Findl O. Capsular fibrosis: a review of prevention methods and management. Eye (Lond) 2020;34:256-62. [Crossref] [PubMed]
- Li J, Liu Z, Wang R, et al. Accuracy of intraocular lens power calculations in paediatric eyes. Clin Exp Ophthalmol 2020;48:301-10. [Crossref] [PubMed]
- Liu ZZ, Long EP, Lin DR, et al. Dynamic profile of ocular refraction in pediatric cataract patients after lens surgeries. Int J Ophthalmol 2019;12:1839-47. [Crossref] [PubMed]
- Solebo AL, Cumberland P, Rahi JS, et al. 5-year outcomes after primary intraocular lens implantation in children aged 2 years or younger with congenital or infantile cataract: findings from the IoLunder2 prospective inception cohort study. Lancet Child Adolesc Health 2018;2:863-71. [Crossref] [PubMed]
- Servick K. Stem cell approach for cataracts challenged. Science 2017;356:1318-9. [Crossref] [PubMed]
- Lin H, Ouyang H, Zhu J, et al. Lens regeneration using endogenous stem cells with gain of visual function. Nature 2016;531:323-8. [Crossref] [PubMed]
- Lyu L, Whitcomb EA, Jiang S, et al. Unfolded-protein response-associated stabilization of p27(Cdkn1b) interferes with lens fiber cell denucleation, leading to cataract. FASEB J 2016;30:1087-95. [Crossref] [PubMed]
- Chaffee BR, Shang F, Chang ML, et al. Nuclear removal during terminal lens fiber cell differentiation requires CDK1 activity: appropriating mitosis-related nuclear disassembly. Development 2014;141:3388-98. [Crossref] [PubMed]
- Gwon A, Gruber L. Engineering the crystalline lens with a biodegradable or non-degradable scaffold. Exp Eye Res 2010;91:220-8. [Crossref] [PubMed]
- Tan X, Liu Z, Zhu Y, et al. The Fate of In Situ Lens Regeneration is Determined by Capsulorhexis Size. Curr Mol Med 2017;17:270-9. [Crossref] [PubMed]
- Tan X, Lin H, Lin Z, et al. Capsular Outcomes After Pediatric Cataract Surgery Without Intraocular Lens Implantation: Qualitative Classification and Quantitative Measurement. Medicine (Baltimore) 2016;95:e2993. [Crossref] [PubMed]
- Tan X, Zhu Y, Chen C, et al. Sprouty2 Suppresses Epithelial-Mesenchymal Transition of Human Lens Epithelial Cells through Blockade of Smad2 and ERK1/2 Pathways. PLoS One 2016;11:e0159275. [Crossref] [PubMed]
- Gwon A, Kuszak J, Gruber LJ. Intralenticular implant study in pigmented rabbits: opacity lensmeter assessment. J Cataract Refract Surg 1999;25:268-77. [Crossref] [PubMed]
- Maki N, Tsonis PA, Agata K J, et al. Changes in global histone modifications during dedifferentiation in newt lens regeneration. Mol Vis 2010;16:1893-7. [PubMed]
- Maki N, Martinson J, Nishimura O, et al. Expression profiles during dedifferentiation in newt lens regeneration revealed by expressed sequence tags. Mol Vis 2010;16:72-8. [PubMed]
- Maki N, Suetsugu-Maki R, Sano S, et al. Oocyte-type linker histone B4 is required for transdifferentiation of somatic cells in vivo. FASEB J 2010;24:3462-7. [Crossref] [PubMed]
- Disatham J, Chauss D, Gheyas R, et al. Lens differentiation is characterized by stage-specific changes in chromatin accessibility correlating with differentiation state-specific gene expression. Dev Biol 2019;453:86-104. [Crossref] [PubMed]
- Klemm SL, Shipony Z, Greenleaf WJ. Chromatin accessibility and the regulatory epigenome. Nat Rev Genet 2019;20:207-20. [Crossref] [PubMed]
- Zhao Y, Zheng D, Cvekl AJ. Profiling of chromatin accessibility and identification of general cis-regulatory mechanisms that control two ocular lens differentiation pathways. Epigenetics Chromatin 2019;12:27. [Crossref] [PubMed]
- Imokawa Y, Brockes JP. Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Current biology 2003;13:877-81. [Crossref] [PubMed]
- Godwin JW, Liem KF, Brockes JP. Tissue factor expression in newt iris coincides with thrombin activation and lens regeneration. Mech Dev 2010;127:321-8. [Crossref] [PubMed]
- McDevitt DS, Brahma SK, Courtois Y, et al. Fibroblast growth factor receptors and regeneration of the eye lens. Dev Dyn 1997;208:220-6. [Crossref] [PubMed]
- Del Rio-Tsonis K, Jung JC, Chiu IM, et al. Conservation of fibroblast growth factor function in lens regeneration. Proc Natl Acad Sci USA 1997;94:13701-6. [Crossref] [PubMed]
- Hayashi T, Mizuno N, Ueda Y, et al. FGF2 triggers iris-derived lens regeneration in newt eye. Mech Dev 2004;121:519-26. [Crossref] [PubMed]
- Fukui L, Henry JJ. FGF signaling is required for lens regeneration in Xenopus laevis. Biol Bull 2011;221:137-45. [Crossref] [PubMed]
- Del Rio-Tsonis K, Trombley MT, McMahon G, et al. Regulation of lens regeneration by fibroblast growth factor receptor 1. Dev Dyn 1998;213:140-6. [Crossref] [PubMed]
- Stone LS. Inhibition of lens regeneration in newt eyes by isolating the dorsal iris from the neural retina. Anat Rec 1958;131:151-71. [Crossref] [PubMed]
- Stone LS. Lens regeneration in adult newt eyes related to retina pigment cells and the neural factor. J Exp Zool 1958;139:69-83. [Crossref] [PubMed]
- Thein T, de Melo J, Zibetti C, et al. Control of lens development by Lhx2-regulated neuroretinal FGFs. Development 2016;143:3994-4002. [Crossref] [PubMed]
- Pathania M, Wang Y, Simirskii VN, et al. β1-integrin controls cell fate specification in early lens development. Differentiation 2016;92:133-47. [Crossref] [PubMed]
- Dawes LJ, Shelley EJ, McAvoy JW, et al. A role for Hippo/YAP-signaling in FGF-induced lens epithelial cell proliferation and fibre differentiation. Exp Eye Res 2018;169:122-33. [Crossref] [PubMed]
- Cvekl A, Zhang X. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet 2017;33:677-702. [Crossref] [PubMed]
- Audette DS, Anand D, So T, et al. Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development 2016;143:318-28. [Crossref] [PubMed]
- Collins TN, Mao Y, Li H, et al. Crk proteins transduce FGF signaling to promote lens fiber cell elongation. Elife 2018;7:e32586. [Crossref] [PubMed]
- Li H, Mao Y, Bouaziz M, et al. Lens differentiation is controlled by the balance between PDGF and FGF signaling. PLoS Biol 2019;17:e3000133. [Crossref] [PubMed]
- Cvekl A, Ashery-Padan R. The cellular and molecular mechanisms of vertebrate lens development. Development 2014;141:4432-47. [Crossref] [PubMed]
- Madhavan M, Haynes TL, Frisch NC, et al. The role of Pax-6 in lens regeneration. Proc Natl Acad Sci USA 2006;103:14848-53. [Crossref] [PubMed]
- Grogg MW, Call MK, Okamoto M, et al. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature 2005;438:858-62. [Crossref] [PubMed]
- Kamachi Y, Uchikawa M, Tanouchi A, et al. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev 2001;15:1272-86. [Crossref] [PubMed]
- Smith AN, Miller LA, Radice G, et al. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development 2009;136:2977-85. [Crossref] [PubMed]
- Duncan MK, Cui W, Oh DJ, et al. Prox1 is differentially localized during lens development. Mech Dev 2002;112:195-8. [Crossref] [PubMed]
- Huang Y, Xie L. Expression of transcription factors and crystallin proteins during rat lens regeneration. Mol Vis 2010;16:341-52. [PubMed]
- Khan SY, Vasanth S, Kabir F, et al. FOXE3 contributes to Peters anomaly through transcriptional regulation of an autophagy-associated protein termed DNAJB1. Nat Commun 2016;7:10953. [Crossref] [PubMed]
- Anand D, Agrawal SA, Slavotinek A, et al. Mutation update of transcription factor genes FOXE3, HSF4, MAF, and PITX3 causing cataracts and other developmental ocular defects. Hum Mutat 2018;39:471-94. [Crossref] [PubMed]
- Krall M, Htun S, Anand D, et al. A zebrafish model of foxe3 deficiency demonstrates lens and eye defects with dysregulation of key genes involved in cataract formation in humans. Hum Genet 2018;137:315-28. [Crossref] [PubMed]
- Cui X, Feng R, Wang J, et al. Heat shock factor 4 regulates lysosome activity by modulating the αB-crystallin-ATP6V1A-mTOR complex in ocular lens. Biochim Biophys Acta Gen Subj 2020;1864:129496. [Crossref] [PubMed]
- Zhao Y, Zheng D, Cvekl A. A comprehensive spatial-temporal transcriptomic analysis of differentiating nascent mouse lens epithelial and fiber cells. Exp Eye Res 2018;175:56-72. [Crossref] [PubMed]
- Si N, Song Z, Meng X, et al. A novel MAF missense mutation leads to congenital nuclear cataract by impacting the transactivation of crystallin and noncrystallin genes. Gene 2019;692:113-8. [Crossref] [PubMed]
- Tsonis PA, Trombley MT, Rowland T, et al. Role of retinoic acid in lens regeneration. Dev Dyn 2000;219:588-93. [Crossref] [PubMed]
- Cvekl A, Wang WL. Retinoic acid signaling in mammalian eye development. Exp Eye Res 2009;89:280-91. [Crossref] [PubMed]
- Blum N, Begemann G. The roles of endogenous retinoid signaling in organ and appendage regeneration. Cell Mol Life Sci 2013;70:3907-27. [Crossref] [PubMed]
- Thomas AG, Henry JJ. Retinoic acid regulation by CYP26 in vertebrate lens regeneration. Dev Biol 2014;386:291-301. [Crossref] [PubMed]
- Hayashi T, Mizuno N, Takada R, et al. Determinative role of Wnt signals in dorsal iris-derived lens regeneration in newt eye. Mech Dev 2006;123:793-800. [Crossref] [PubMed]
- Hamilton PW, Sun Y, Henry JJ, et al. Lens regeneration from the cornea requires suppression of Wnt/β-catenin signaling. Exp Eye Res 2016;145:206-15. [Crossref] [PubMed]
- Tsonis PA, Vergara MN, Spence JR, et al. A novel role of the hedgehog pathway in lens regeneration. Dev Biol 2004;267:450-61. [Crossref] [PubMed]
- Day RC, Beck CW. Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signalling and involves upregulation of Wnt signalling. BMC Dev Biol 2011;11:54. [Crossref] [PubMed]
- Murphy P, Kabir MH, Srivastava T, et al. Light-focusing human micro-lenses generated from pluripotent stem cells model lens development and drug-induced cataract in vitro. Development 2018;145:dev155838. [Crossref] [PubMed]
- Yang C, Yang Y, Brennan L, et al. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions. FASEB J 2010;24:3274-83. [Crossref] [PubMed]
- Medvedovic M, Tomlinson CR, Call MK, et al. Gene expression and discovery during lens regeneration in mouse: regulation of epithelial to mesenchymal transition and lens differentiation. Mol Vis 2006;12:422-40. [PubMed]
- Shaw TJ, Martin P. Wound repair: a showcase for cell plasticity and migration. Curr Opin Cell Biol 2016;42:29-37. [Crossref] [PubMed]
- Arnoux V, Nassour M, L'Helgoualc'h A, et al. Erk5 controls Slug expression and keratinocyte activation during wound healing. Mol Biol Cell 2008;19:4738-49. [Crossref] [PubMed]
- Aurora AB, Olson EN. Immune modulation of stem cells and regeneration. Cell Stem Cell 2014;15:14-25. [Crossref] [PubMed]
- Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep 2010;2:3. [Crossref] [PubMed]
- Khaja WA, Verma M, Shoss BL, et al. Ophthalmology YKJ. Visual axis opacification in children. Ophthalmology 2011;118:224-5. [Crossref] [PubMed]
- Apple DJ, Escobar-Gomez M, Zaugg B, et al. Modern cataract surgery: unfinished business and unanswered questions. Surv Ophthalmol 2011;56:S3-S53. [Crossref] [PubMed]
- Rønbeck M, Kugelberg M. Posterior capsule opacification with 3 intraocular lenses: 12-year prospective study. J Cataract Refract Surg 2014;40:70-6. [Crossref] [PubMed]
- Abnave P, Ghigo E. Role of the immune system in regeneration and its dynamic interplay with adult stem cells. Semin Cell Dev Biol 2019;87:160-8. [Crossref] [PubMed]
- Julier Z, Park AJ, Briquez PS, et al. Promoting tissue regeneration by modulating the immune system. Acta Biomater 2017;53:13-28. [Crossref] [PubMed]
- Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest 2014;124:1382-92. [Crossref] [PubMed]
- Haider N, Boscá L, Zandbergen HR, et al. Transition of Macrophages to Fibroblast-Like Cells in Healing Myocardial Infarction. J Am Coll Cardiol 2019;74:3124-35. [Crossref] [PubMed]
- Logan CM, Bowen CJ, Menko AS. Induction of Immune Surveillance of the Dysmorphogenic Lens. Sci Rep 2017;7:16235. [Crossref] [PubMed]
- Jiang J, Shihan MH, Wang Y, et al. Lens Epithelial Cells Initiate an Inflammatory Response Following Cataract Surgery. Invest Ophthalmol Vis Sci 2018;59:4986-97. [Crossref] [PubMed]
- Wu X, Liu Z, Wang D, et al. Preoperative profile of inflammatory factors in aqueous humor correlates with postoperative inflammatory response in patients with congenital cataract. Mol Vis 2018;24:414-24. [PubMed]
- Ikai C, Okamoto M. Reduced macrophage phagocytic activity in Wolffian lens regeneration of the newt after nickel subsulfide administration. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1998;119:81-8. [Crossref] [PubMed]
- Reyer RW. Macrophage invasion and phagocytic activity during lens regeneration from the iris epithelium in newts. Am J Anat 1990;188:329-44. [Crossref] [PubMed]
- Kanao T, Miyachi Y. Lymphangiogenesis promotes lens destruction and subsequent lens regeneration in the newt eyeball, and both processes can be accelerated by transplantation of dendritic cells. Dev Biol 2006;290:118-24. [Crossref] [PubMed]
- Neumayer T, Buehl W, Findl O. Effect of topical prednisolone and diclofenac on the short-term change in morphology of posterior capsular opacification. Am J Ophthalmol 2006;142:550-6. [Crossref] [PubMed]
- Doganay S, Turkoz Y, Evereklioglu C, et al. Use of caffeic acid phenethyl ester to prevent sodium-selenite-induced cataract in rat eyes. J Cataract Refract Surg 2002;28:1457-62. [Crossref] [PubMed]
- Tarsio JF, Kelleher PJ, Tarsio M, et al. Inhibition of cell proliferation on lens capsules by 4197X-ricin A immunoconjugate. J Cataract Refract Surg 1997;23:260-6. [Crossref] [PubMed]
- Wride MA. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos Trans R Soc Lond B Biol Sci 2011;366:1219-33. [Crossref] [PubMed]
- He S, Limi S, McGreal RS, et al. Chromatin remodeling enzyme Snf2h regulates embryonic lens differentiation and denucleation. Development 2016;143:1937-47. [Crossref] [PubMed]
- Cao L, Liu J, Pu J, et al. Endogenous bioelectric currents promote differentiation of the mammalian lens. J Cell Physiol 2018;233:2202-12. [Crossref] [PubMed]
- Dawes LJ, Sugiyama Y, Tanedo AS, et al. Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Invest Ophthalmol Vis Sci 2013;54:1582-90. [Crossref] [PubMed]
- Dawes LJ, Sugiyama Y, Lovicu FJ, et al. Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism. Dev Biol 2014;385:291-303. [Crossref] [PubMed]