Factors influencing the length of stay after mediastinal tumor resection in the setting of an enhanced recovery after surgery (ERAS)-TUBELESS protocol
Introduction
Mediastinal tumors represent a wide diversity of disease states. Although more than two-thirds of mediastinal tumors are benign, some of them cause life-threatening symptoms by infection, enlargement, and invasion of intrathoracic organs requiring surgical treatment (1). Compared with conventional thoracotomy, minimally invasive surgery has been associated with a shorter length of hospital stay, fewer complications, less pain and a better quality of life (2,3). Representative examples are the lateral intercostal approach in video-assisted thoracoscopic surgery (VATS) tumor resection and robot-assisted tumor resection, the cervical incision in transcervical tumor resection and the infrasternal approach (4).
Enhanced recovery after surgery (ERAS) is a multimodal, multidisciplinary, scientific approach to the perioperative care of the surgical patient. ERAS protocol process implementation involves a team consisting of surgeons, anesthetists, an ERAS coordinator, and staff from units that care for the surgical patient (5). This protocol initially developed in colorectal surgery but has been shown to improve outcomes in almost all major surgical specialties including thoracic surgery. With the progress of minimally invasive thoracoscopic and thoracic anesthesia techniques, ERAS protocol has been also developed in the field of thoracic surgery and length of stay has been significantly reduced (6,7). Our center has advocated and promoted spontaneous ventilation VATS (SV-VATS) since 2011 (8). In addition to the adoption of SV-VATS, avoidance of any invasive tool including urinary catheter, central venous lines and early removal of the chest tube after thoracic surgery or even removal of the tube at end-procedure, which might be defined as tubeless SV-VATS. Tubeless SV-VATS further improved the ERAS protocol and associated with a decreased length of stay after VATS tumor resection (LOS) (9).
Prolonged LOS is a substantial driver of cost and hospital-acquired complications (10). The precise identification of patients who need more rehabilitation time and more extensive care can optimize rehabilitation and discharge planning. Thus, reducing the cost to the hospital and the health care system and offering better an outcome to patients. However, there is no special study to describe the risk factors that prolong LOS. In this study, we aim to identify the risk factors that are associated with an increased LOS after mediastinal tumor resection in the setting of an ERAS-TUBELESS protocol. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-287).
Methods
Study design and patients
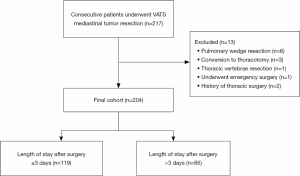
This was a retrospective study in the first affiliated hospital of Guangzhou Medical University between December 2015 and November 2018. For the current analysis, all consecutive patients underwent VATS mediastinal tumor resection were selected to extract detailed information through electronic medical records. Patients were excluded if they were discharged without meeting the discharge criteria. Patients who converted from VATS to open thoracotomy or had a history of thoracic surgery were excluded. What’s more, patients who underwent emergency surgery or performed non-mediastinal surgery simultaneously were further excluded to maintain cohort homogeneity. The primary outcome was LOS. The LOS was defined as the number of nights after the operation in the hospital. LOS was dichotomized by performing a median split. This cohort was further divided into two subgroups based on LOS (Figure 1). A LOS greater than the median was considered a prolonged LOS. Potential risk factors associated with prolonged LOS were obtained from electronic medical records.
This cohort was further divided into two subgroups based on LOS. The choice of which anesthesia and operation procedure was based on anesthetist, surgeon’s discretion and patients’ wishes. All patients had signed the consent before the surgery. The National Key R&D Program of China evaluated the study. All data involved in this study were collected retrospectively and didn’t disclose identity information, which was not required the statement of ethics approval.
Surgical technique
Surgical approaches included VATS tumor resection via a lateral intercostal approach and a subxiphoid approach. All surgeries were performed by 3 surgical teams, each team had one chief surgeon. The choice of which surgical procedure was based on the surgeon’s discretion and patients’ wishes. Anesthesia procedures were the same as described by Liang et al. (11). All patients had signed the operation consent before the surgery. The lateral intercostal approach was previously described by Jiang et al. (12). Briefly, the patient was tilted 30° lateral in a semisupine position with a roll under the shoulder and the ipsilateral arm held abducted over a padded L-screen to expose the axilla for port placement. Uniport VATS technique was created one incision. A 30° angled camera and endoscopic instruments were placed in the 2- to 4-cm port. Two-port VATS was created two-incision. One 2- to 3-cm port for surgical procedure and one 1-cm port placed in the lower lateral for using a 30° angled camera. Three-port VATS was created three 1-cm ports. All specimens were safely removed via a specimen bag. All specimens were safely removed via a specimen bag by enlarging one of the anterior port incisions. Any bleeding or air leak was managed by reinforcement sutures using 4/0 PROLENE (Ethicon, Somerville, NJ, USA) or application of sealants such as Biopaper (Datsing Bio-Tech Co Ltd., Beijing, China). Some patients placed a 24-F chest tube at the end of the operation.
The subxiphoid approach was briefly described as below. The patient was placed in a supine position with the legs open. A 2-cm observation port which placed a 30° angled camera was made under the inferior edge of the xiphoid. Skin, subcutaneous fat and the rectus abdominis muscle was separated along the costal margin. In order to enlarge the retrosternal space, carbon dioxide was insufflated into the mediastinum in some patients. Two 1-cm extra pleural thoracic ports which placed ultrasonic scalpel and grasping forceps were created under the bilateral costal arches. The tumor was dissociated and removed safely. Not all patients left the drainage tube at the end of the operation.
ERAS
Our center firstly reported the SV-VATS strategy in 2011 (8). Since then our center has implemented the ERAS-TUBELESS protocol and constantly improved it. The ERAS-TUBELESS protocol includes patient education, preoperative management, anesthesia, surgery procedure, postoperative and postoperative complications management. Our center attached great importance to early postoperative ambulation, weight management, avoidance of muscle relaxants, regional anesthesia, pain management, and early removal of chest tube after surgery or even removal of the tube at end-procedure. Improving pulmonary function through weight management (13). The patients are adjusted to the optimal state to create conditions for accurate anesthesia and precise surgical. At the foundation of this protocol, tubeless SV-VATS promotes thoracic day surgery.
Postoperative management
Respiratory rate, heart rate, blood pressure, and oxygen saturation were measured after surgery. Routine blood, D-dimer and arterial blood gas analysis, X-ray chest plain film or B-mode ultrasonographic scanning were checked after back to the ward or ICU from Post Anesthesia Care Unit. Follow the multimodal analgesia principle to manage postoperative pain. Patients were encouraged to become ambulatory as soon as possible after surgery. The criteria for chest tube removal were as follows: the chest tube can be removed when X-ray chest plain film reveals the remaining lungs were completely re-expanded, and there was no obvious air leak, active bleeding, and total drainage less than 100 mL in 24 hours. Patients discharged criteria were as follows: normal vital signs, no complications requiring in-hospital treatment, no residual abundant pleural effusion, lung re-expansion >70% after the drainage tube removal.
Data collection and statistical analyses
Risk factors influenced LOS in the analysis were divided into patient-related risk factors and procedure-related risk factors. Patient-related risk factors included the following: age, gender, body mass index (BMI), pulmonary function, symptom, comorbidity, and American Society of Anesthesiologists (ASA) status class. Procedure-related risk factors included anesthesia method, surgeon, tumor location, tumor size, tumor histology, location of the incision (operative method), operation time, intraoperative blood loss, drainage tube (the number of patients placed drainage tube), postoperative D-dimer, postoperative white blood cell (WBC), postoperative systemic immune-inflammation index (SII), analgesic drugs, complications and unplanned situations in surgery. The SII was calculated by using the following formula: SII = platelet count × neutrophil count/lymphocyte count.
Data were presented as mean value with standard deviation or median with interquartile range (IQR) for continuous variables, and percentages for categorical variables. Continuous variables with normal distribution were compared using t-test, whereas those without normal distribution were compared using the Mann-Whitney U-test. Categorical variables were compared using the Pearson’s χ2 test or Fisher’s exact test. Variables with a P value of <0.05 in the univariable analysis were selected as independent variables in a multivariable logistic regression analysis. The models’ fit was assessed using a Hosmer-Lemeshow test. All P values were bilaterally distributed, and P<0.05 was considered statistically significant. SPSS software (SPSS version 25.0; IBM Corp, Armonk, NY, USA) was used for all statistical evaluations.
Results
Patients characteristics
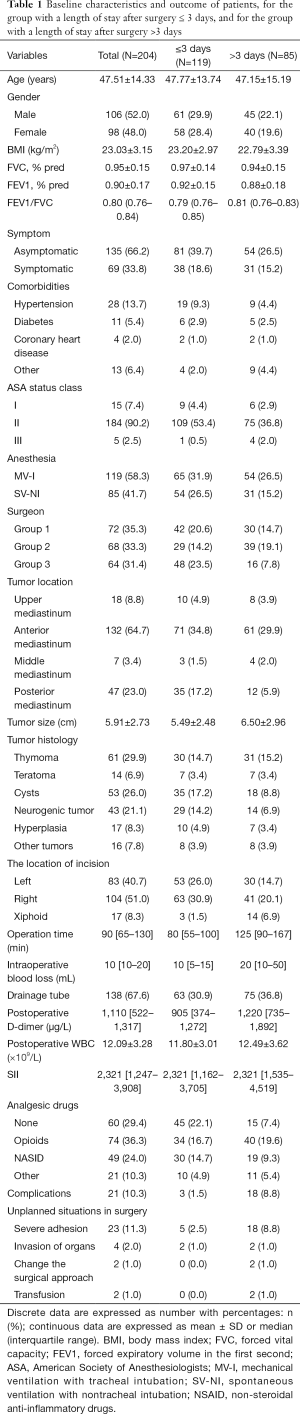
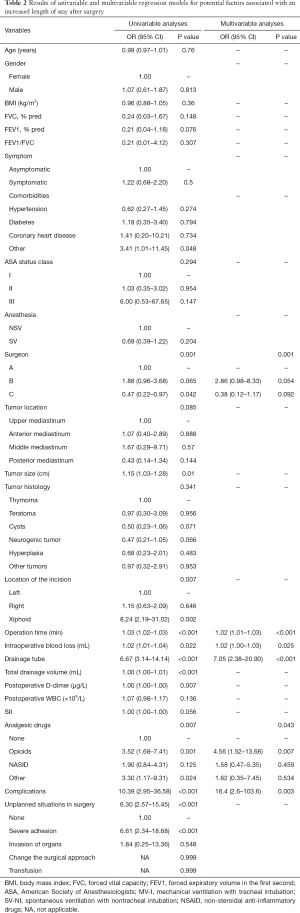
A total of 204 patients between December 2015 and November 2018 were consecutively included in the analysis, the median LOS for the entire cohort was 3 days (IQR, 2–5 days) with a mean of 3.5 days (SD, 2.4). A total of 85 (41.67%) patients had a LOS of more than 3 days. The median LOS for LOS ≤3 days group and LOS >3 days group were 2 days (IQR, 2–3 days) and 5 days (IQR, 4–7 days), respectively. The mean age of the whole group was 47.51±14.33 years. The demographics of patients were shown in Table 1. The results of the univariable and multivariable models were presented in Table 2.
Full table
Full table
Patient-related risk factors
Patient-related risk factors included age, gender, BMI, pulmonary function, symptom, comorbidity, and ASA status class. As shown in Table 2, univariate analysis of all the patient-related risk factors had no significantly associated with a prolonged LOS except the comorbidities of other. Other comorbidities included gout, tuberculosis, hepatitis B carriers, asthma and history of cancer. Patients in the LOS >3 days group had more other comorbidities [9 patients (4.4%) vs. 4 patients (2.0%); P=0.048; odds ratio (OR), 3.41; 95% CI, 1.01–11.45]. However, the comorbidities of other had no significant difference in multivariate analysis.
Procedure-related risk factors
Univariate analysis of all the procedure-related risk factors revealed tumor size, location of the incision, operation time, intraoperative blood loss, drainage tube, postoperative D-dimer, analgesic drugs, complications, and intraoperative unplanned situations to be significantly associated with a prolonged LOS. In the multivariate model, procedure-related risk factors that were significantly associated with a prolonged LOS were surgeon (P=0.001), operation time [80 min (IQR, 55–100 min) vs. 125 min (IQR, 90–167 min); P<0.001; OR, 1.02; 95% CI, 1.01–1.03], intraoperative blood loss [10 mL (IQR, 5–15 mL) vs. 20 mL (IQR, 10–50 mL); P=0.025; OR, 1.02; 95% CI, 1.00–1.03], drainage tube [63 patients (30.9%) vs. 75 patients (36.8%); P<0.001; OR, 7.05; 95% CI, 2.38–20.90], analgesic drugs (P=0.043) and complications [3 patients (1.5%) vs. 18 patients (8.8%); P=0.003; OR, 16.4; 95% CI, 2.6–103.6]. Complications included pleural effusion, air leakage, pneumonia, myasthenia, and hoarseness. The analgesic drugs of other included tramadol and rotundine.
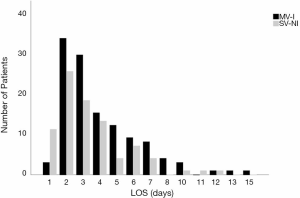
In the univariate analysis, anesthesia had no significant correlation [spontaneous ventilation with nontracheal intubation (SV-NI) vs. mechanical ventilation with tracheal intubation (MV-I); P=0.204; OR, 0.69; 95% CI, 0.39–1.22] with a prolonged LOS (LOS ≤3 days vs. LOS >3 days). Figure 2 displayed LOS for patients underwent MV-I and SV-NI. Histogram demonstrating the distribution of LOS among patients underwent different anesthesia procedures. Patients who underwent MV-I and SV-NI demonstrated a similar distribution of LOS. However, the SV-NI group had more patients than the MV-I group on the first day of LOS and had fewer patients on the other days of LOS. Mann-Whitney U-test show a significant difference (P=0.025) between anesthesia and LOS (days). When the whole cohort was divided into LOS ≤1 day group and LOS >1 day group, there was a significant association with anesthesia [SV-NI vs. MV-I; P=0.009; OR, 0.17; 95% CI, 0.05–0.64], and this remained an independent risk factor in multivariate analysis [SV-NI vs. MV-I; P=0.017; OR, 0.16; 95% CI, 0.04–0.72].
Discussion
Decreasing the length of stay after surgery to a single day had become the impetus to improve thoracic surgery. Hence, it is imperative to understand the drivers of LOS and how to identify candidates for a prolonged hospital stay. The most important clinically relevant finding of the present study was that an increased LOS was associated with procedure-related risk factors including surgeon, operation time, intraoperative blood loss, drainage tube, analgesic drugs, and complications. Although patient-related risk factors have been shown to influence LOS, all of the patient-related risk factors were not independent risk factors. Anesthesia was associated with early discharge (LOS ≤1 day), anesthesia with spontaneous ventilation promoted thoracic day surgery and rapid recovery after surgery.
The finding that the primary predictors of LOS were procedure-related risk factors rather than patient-related risk factors further supports the need to optimize and adhere to ERAS-TUBELESS protocols. The mean LOS in our study was 3.8 days and the median LOS was 3 days, which is shorter than some other studies (14-16). Our center has advocated tubeless since 2011. Avoidance of any invasive tool included tracheal intubation, urethral catheter, central venous catheter and early removal of the chest tube after thoracic surgery or even removal of the tube at end-procedure. In the present study, the drainage tube was associated with a prolonged LOS. Similar studies reported that chest tube can cause various complications including the risk of infection, pain and prolonged hospital stay, removed chest tube as soon as possible can significantly shorten the length of stay and reduced costs (17-20). An expert consensus proposed that any unnecessary use of the chest tube should be avoided (9).
One interesting finding was that anesthesia had no significant correction with a prolonged LOS (LOS ≤3 days vs. LOS >3 days). However, Figure 2 show that the SV-NI group had more patients than the MV-I group on the first day of LOS and had fewer patients on the other days of LOS. What’s more, a previous study in our center found that LOS was shorter in SV-VATS mediastinal tumor resection (11). So divided the cohort into LOS ≤1 day group and LOS >1 day group, logistic regression show that anesthesia was an independent risk factor. Anesthesia with nontracheal intubation avoided muscle relaxants, intubation-related and mechanical ventilation-associated complications (21). The avoidance of muscle relaxants may prevent adverse respiratory effects caused by residual muscle block, ranging from diaphragmatic dysfunctions, weakness of upper airway muscles and skeletal muscle, and thus accelerate recovery (22). Nontracheal intubation caused less damage to the trachea and less oxidative response owing to intubation so as to shorten the length of stay after surgery. In the present study, SV-NI contributed to early discharge and day surgery.
Appropriate analgesia is crucial after thoracic surgery and a multimodal therapeutic strategy that aims toward enhanced recovery and shortened length of stay. Our center managed postoperative pain through the multimodal analgesia principle. The paravertebral block was applied to keep patient spontaneous ventilation and maintain the operation stable. Several reports have shown that paravertebral block offers good pain relief, less nausea, and vomiting and contributes to enhanced recovery after thoracic surgery (23,24). What’s more, early postoperative ambulation can reduce the length of stay (6,7). And immobility after thoracic surgery is common and largely due to pain, nausea, drowsiness, continued chest drainage (25). In this analysis, opioids were significantly associated with prolonged LOS. As described in a previous study, the SV-NI technique significantly decreased the need for prescription of opioids (11). What’s more, early removal of the chest tube or no placement of chest tube can effectively reduce the use of analgesics (20). Inflammation is the human reaction to endogenous or exogenous injury and playing an important role in the growth of tumors(26). SII is an objective marker that reflects host inflammation, immune response status, and prognosis (27-29). But SII wasn’t an independent risk factor of prolonged length of stay in this analysis. In addition, surgical-related risk factors of prolonged length of stay included operation time and intraoperative blood loss. Although it was hard to improve these factors, it can optimize rehabilitation and discharge planning.
Study limitations
There are noted limitations to this investigation. Firstly, the study was retrospective and collected based on historical controls. Secondly, we performed a median split to dichotomize LOS. No research has reported the optimal cut-off point. In the absence of a prior cut-off point, the common approach is to take the cohort median. Different cut-off points probably have different results. Third, our thoracic center had 3 units and each unit had different surgeons. The management and decision were somewhat different in each unit. And surgeon was also a manager of ERAS-TUBELESS protocol. So, the risk factor of surgeon was complex.
Conclusions
In the setting of an ERAS-TUBELESS protocol, understanding risk factors that affect outcomes after VATS mediastinal tumor resection provides the opportunity to influence them favorably to optimize care. Overall, the main drivers of LOS were procedure-related factors including surgeon, operation time, intraoperative blood loss, drainage tube, analgesic drugs, and complications. Anesthesia with spontaneous ventilation was associated with early discharge (LOS ≤1 day) and thus promoted thoracic day surgery.
Acknowledgments
Funding: This work was supported by the grant 2016YFC0905400 from the National Key R&D Program of China; China National Science Foundation (grant No. 81871893 & No. 81501996); Key Project of Guangzhou Scientific Research Project (grant No. 201804020030); High-level University Construction Project of Guangzhou Medical University (grant No. 20182737, 201721007, 201715907, 2017160107); National Key R&D Program (grant No. 2017YFC0907903 & 2017YFC0112704).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-287
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-287
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-287
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-287). JH serves as an unpaid Editor-in-Chief of Annals of Translational Medicine from Jun 2019 to May 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the First Affiliated Hospital of Guangzhou Medical University and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]
- He J, Liu J, Zhu C, et al. Expert consensus on tubeless video-assisted thoracoscopic surgery (Guangzhou). J Thorac Dis 2019;11:4101-8. [Crossref] [PubMed]
- Brunelli A, Drosos P, Dinesh P, et al. The Severity of Complications Is Associated With Postoperative Costs After Lung Resection. Ann Thorac Surg 2017;103:1641-6. [Crossref] [PubMed]
- Liang H, Liu J, Wu S, et al. Nonintubated Spontaneous Ventilation Offers Better Short-term Outcome for Mediastinal Tumor Surgery. Ann Thorac Surg 2019;108:1045-51. [Crossref] [PubMed]
- Jiang L, Depypere L, Rocco G, et al. Spontaneous ventilation thoracoscopic thymectomy without muscle relaxant for myasthenia gravis: Comparison with "standard" thoracoscopic thymectomy. J Thorac Cardiovasc Surg 2018;155:1882-9.e3. [Crossref] [PubMed]
- Forno E, Han YY, Mullen J, et al. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J Allergy Clin Immunol Pract 2018;6:570-581.e10. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Wu CF, Gonzalez-Rivas D, Wen CT, et al. Comparative Short-Term Clinical Outcomes of Mediastinum Tumor Excision Performed by Conventional VATS and Single-Port VATS: Is It Worthwhile? Medicine (Baltimore) 2015;94:e1975. [Crossref] [PubMed]
- Zhang L, Li M, Jiang F, et al. Subxiphoid versus lateral intercostal approaches thoracoscopic thymectomy for non-myasthenic early-stage thymoma: A propensity score -matched analysis. Int J Surg 2019;67:13-7. [Crossref] [PubMed]
- McKenna RJ, Mahtabifard A, Pickens A, et al. Fast-tracking after video-assisted thoracoscopic surgery lobectomy, segmentectomy, and pneumonectomy. Ann Thorac Surg 2007;84:1663-7; discussion 1667-8.
- Nakanishi R, Fujino Y, Yamashita T, et al. A prospective study of the association between drainage volume within 24 hours after thoracoscopic lobectomy and postoperative morbidity. J Thorac Cardiovasc Surg 2009;137:1394-9. [Crossref] [PubMed]
- Satherley LK, Luckraz H, Rammohan KS, et al. Routine placement of an intercostal chest drain during video-assisted thoracoscopic surgical lung biopsy unnecessarily prolongs in-hospital length of stay in selected patients. Eur J Cardiothorac Surg 2009;36:737-40. [Crossref] [PubMed]
- Liu YW, Chen HW, Lee JY, et al. Is a Chest Tube Necessary after Video-Assisted Thoracoscopic Mediastinal Tumor Resection? Thorac Cardiovasc Surg 2019. Epub ahead of print. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Komatsu T, Kino A, Inoue M, et al. Paravertebral block for video-assisted thoracoscopic surgery: analgesic effectiveness and role in fast-track surgery. Int J Surg 2014;12:936-9. [Crossref] [PubMed]
- Rivedal DD, Nayar HS, Israel JS, et al. Paravertebral block associated with decreased opioid use and less nausea and vomiting after reduction mammaplasty. J Surg Res 2018;228:307-13. [Crossref] [PubMed]
- Agostini PJ, Naidu B, Rajesh P, et al. Potentially modifiable factors contribute to limitation in physical activity following thoracotomy and lung resection: a prospective observational study. J Cardiothorac Surg 2014;9:128. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-e503. [Crossref] [PubMed]
- Huang H, Liu Q, Zhu L, et al. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci Rep 2019;9:3284. [Crossref] [PubMed]
- Yang R, Chang Q, Meng X, et al. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer 2018;9:3295-302. [Crossref] [PubMed]
- Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 2015;15:350. [Crossref] [PubMed]