Artesunate induces apoptosis and inhibits the proliferation, stemness, and tumorigenesis of leukemia
Introduction
Leukemia, which is characterized by tumors of the hematopoietic system, is one of the main malignancies endangering human health. Its incidence among all malignant tumors (liver cancer, stomach cancer, lung cancer, oesophagus cancer, rectum cancer, breast cancer, pancreas cancer, leukaemia, bran cancer and central nervous system cancer and colon cancer) ranks seventh in China (47 cases per 100,000) and sixth in Europe and America (64 cases per 100,000) (1). Chemotherapy and bone marrow transplantation are the main treatment methods for leukemia. Although they have a certain curative effect and prolong the survival of patients, due to the lack of specificity of chemotherapy drugs, they often cause severe side effects to patients, and some patients eventually develop drug-resistant recurrence (2). Therefore, more effective and less adverse therapeutic strategies need to be explored.
Artesunate (Art), a semi-synthetic derivative of artemisinin from Artemisia annual, is a first-line medicine for malaria (3). In the past decade, Art has been tested for its anticancer effects on various types of human malignancies including leukemia (4-6). For instance, Art has been found to induce the apoptosis of acute myeloid leukemia THP-1 cells, myelodysplastic syndrome SKM-1 cells, and HepG2 cells (7-9), and inhibit the cell proliferation of breast cancer cells (10). Leukemia stem cells possess self-renewal capacity and differentiation potential, which play a critical role in leukemia resistance, relapse, and prognosis (11). Stem cells can also confer a unlimited proliferative capacity to cancer cells (12). Art has been shown to kill several kinds of leukemia cells (6,7,13), suggesting its antileukemia effect. However, the mechanism underling this effect is poorly understood.
Therefore, this study aimed to clarify the antileukemia mechanism of Art in regards to the proliferation, stemness, and tumorigenesis of this malignancy. To this end, human acute promyelocyte leukemia HL-60 cells and acute myeloid leukemia KG1a cells were examined in vitro. Then, the expression of MEK/ERK and PI3K/Akt pathway proteins were determined in both Art-treated and non-Art-treated leukemia cells. Finally, a nude mice xenograft model was established in vivo to detect the effects of Art on tumor growth and stemness.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4558).
Methods
Cell culture
Human acute promyelocyte leukemia HL-60 cells and acute myeloid leukemia KG1a cells were purchased from the American Type Culture Collection (ATCC)(Manassas, VA, USA) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), penicillin (100 U/mL, Gibco), and streptomycin (100 µg/mL, Gibco). These cells were maintained at 37 °C in an incubator with 5% CO2. Art was purchased from Sigma-Aldrich and dissolved in phosphate-buffered saline (PBS).
Flow cytometry
HL-60 and KG1a cells were treated with Art (0, 10, 25, and 50 µM) for 24 hours. They were washed with ice-cold phosphate-buffered saline (PBS) before resuspension in Annexin V binding buffer. Then, incubation with fluorescein isothiocyanate (FITC)-conjugated Annexin V antibody (Cell Signaling Technology, Danvers, USA) and propidium iodide took place for 15 min at room temperature. Data analysis was performed using FlowJo (Tree Star, Ashland, OR, USA).
Colony formation assay
The proliferation capacity of HL-60 and KG1a cells was measured by colony formation assay. Then, 2×103 cells were cultured in six-well plates with 2 mL of RPMI 1640 medium containing 10% FBS at 37 °C for 10–14 days, until the colonies were visible to the naked eye. The cells were fixed with methanol and stained with 1% crystal violet for 15 minutes. Then, colony numbers were counted. All experiments were performed in triplicate.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Purified RNAs were extracted from HL-60 and KG1a cells using Trizol reagent (Invitrogen, USA). First-strand cDNA synthesis was performed using a PrimeScript™ RT reagent Kit (TaKaRa). The cDNA synthesis was performed at 37 °C for 60 minutes after heating at 95 °C for 10 minutes. The cDNA was amplified using SYBR Premix Ex Taq™ II (TaKaRa). The qRT-PCR data were analyzed using 2–ΔΔCt method to calculate the relative expression levels of Ki67, survivin and P21. Relative Ki67, survivin and P21. mRNAs were normalized to β-actin. All reactions were performed in triplicate.
Western blotting analysis
HL-60 and KG1a cells were treated with Art (0, 10, 25, and 50 µM) for 48 hours. HL-60 and KG1a cells were collected, and tumor tissues were homogenized. Protein was extracted with radioimmunoprecipitation assay (RIPA) lysis buffer plus cocktail (Sigma Aldrich) and phenylmethanesulfonyl fluoride (PMSF, Sigma Aldrich). Next, 20 µg of protein extract was separated on 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk and then incubated with the primary antibodies(anti-survivin, ab76424; anti-P21, ab188224; anti-cleaved caspase 3, ab2302; anti-Bax, ab32503; anti-Bcl-2, ab59348; anti-Ki67, ab16667; anti-CD44, ab157107; anti-SOX2, ab93689; anti-ALDH1, ab52492; anti-OCT4, ab181557; anti-MEK1, ab96379; Anti-p-MEK1, ab214445; anti-ERK1/2, ab17942; Anti-p-ERK1/2, ab223500; anti-PI3K,ab32089; Anti-p-PI3K, ab182651; anti-Akt, ab235958; Anti-p-AKT, ab8805) on a shaker overnight at 4 °C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (rabbit IgG, 1/1,000 diluted; UK). A β-action antibody (Santa Cruz Biotech) was used as a control. All bands were detected using an enhanced chemiluminescence (ECL) Western blot kit (Amersham Biosciences, UK). The bands were measured with an Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare, UK).
Cell sphere formation assay
First, 0.5×103 cells were seeded in Ultra-Low Attachment six-well plates (Corning, Corning, NY,USA) in the serum-free RPMI 1640 medium supplemented with 20 ng/mL EGF, 20 ng/mL bFGF, N-2 and, 2% B27 (Invitrogen, Carlsbad, CA, USA). After 14 days of culture, microscope images were acquired from the plate. The number and diameters of tumor spheres (diameter >30 µm) were counted from 4 to 6 images for each well. All results were reported relative to the negative control.
Animal experiments
Each of the animal experiments in this study was conducted according to the principles of the NIH Guide for the Care and Use of Laboratory Animals and received approval from The First Affiliated Hospital of Zhengzhou University (No. 20190918). Five-week-old male athymic nude mice were purchased from the Model Animal Research Institute of Zhengzhou University. Approximately 1×107 KG1a cells were injected subcutaneously into the right flank of each nude mouse. The mice were randomly divided into the control and treatment group. The treatment group received 100 mg/kg Art i.p. three times a week for 3 weeks. The mice were humanely killed, and the tumors were photographed.
Immunohistochemistry
Immunohistochemistry for Ki67 and survivin was performed to detect tumor expression. Sections (2 µm) were deparaffinized and pretreated with citrate buffer using a heat-induced epitope retrieval protocol. Endogenous peroxidase was blocked with 20% hydrogen peroxide for 15 minutes at room temperature followed by incubation with anti-Ki67 and anti-survivin for 30 minutes respectively. A biotinylated goat anti-mouse immunoglobulin G secondary antibody (Dako, Denmark) was then applied for 30 minutes. Slides were incubated with peroxidase-conjugated streptavidin reagent (Dako, Denmark) and developed with 3,3’-diaminobenzidine for 5 minutes. The slides were counterstained and dehydrated.
Statistics analysis
The results are expressed as mean values ± standard deviation (SD) or standard error (SE). Statistical comparisons were analyzed by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. A significance level of <0.05 was used for all tests. SPSS-PASW statics software version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all the statistical analyses.
Results
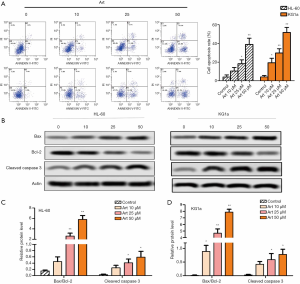
Art promoted apoptosis of leukemia cells
To determine the influence of Art on the apoptosis of leukemia cells, HL-60 and KG1a cells were treated with 0, 10, 25, and 50 µM of Art, and flow cytometry assay was performed. As shown in Figure 1A, 10, 25, and 50 µM Art stimulated cell apoptosis in a dose-dependent manner. To confirm the enhanced apoptosis, Western blotting was conducted. As depicted in Figure 1B,C,D, the protein level of cleaved caspase3 and the ratio of Bax/Bcl-2 were significantly up-regulated by Art in a dose-dependent manner (P<0.05).
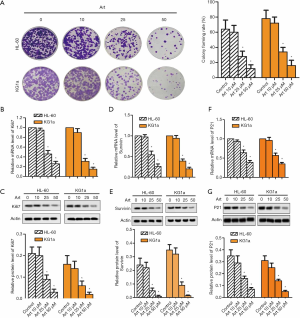
Art impeded proliferation of leukemia cells
To determine the role of Art on proliferation of leukemia cells, HL-60 and KG1a cells were treated as previously described, and colony formation assay was performed. As shown in Figure 2A, 10, 25, and 50 µM of Art suppressed the colony forming rate in both HL-60 and KG1a cells in a dose-dependent manner (P<0.05). Quantitative real-time polymerase chain (qRT-PCR) and Western blotting were applied. The Ki67 expression in both the mRNA level and protein level was significantly reduced by Art in a dose-dependent manner (Figure 2B,C, P<0.05). As depicted in Figure 2D,E, the mRNA level and protein level of survivin were both significantly reduced by Art in a dose-dependent manner (P<0.05). Figure 2F,G shows that mRNA level and protein level of P21 were both significantly reduced by Art in a dose-dependent manner (P<0.05).
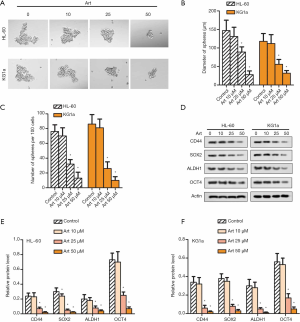
Art suppressed stemness of leukemia cells
To determine the role of Art on stemness of HL-60 and KG1a cells, cell sphere formation assay was performed, and the result was observed under a phase contrast microscope. As shown in Figure 3A, 10, 25, and 50 µM of Art suppressed the cell sphere formation ability in a dose-dependent manner as evidenced by the reduced diameters of the spheres (Figure 3B) and sphere numbers (Figure 3C). Detection of expression of stemness marker proteins was then performed. The expressions of CD44, SOX2, ALDH1 and OCT4 were all inhibited by Art in a dose-dependent manner (Figure 3D,E,F, P<0.05). These results indicated that Art significantly reduced stemness of HL-60 and KG1a leukemia cells.
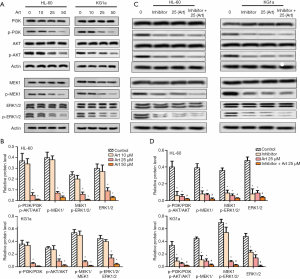
Art inhibited MEK/ERK and PI3K/Akt signaling pathways
MEK/ERK and PI3K/Akt signaling pathways have been shown to play essential roles in leukemia. We thus used Western blot to detect the pathway protein. As shown in Figure 4A,B, the expressions of p-PI3K, p-AKT, p-MEK1, and p-ERK1/2 were all inhibited by Art in a dose-dependent manner in both HL60 and KG1a cells. After an inhibitor was added, the HL60 and KG1a cells was divided into control group, inhibitor group, Art (25 µM) group and Art (25 µM) + inhibitor group (Figure 4C,D). The expressions of p-PI3K, p-AKT, p-MEK1, and p-ERK1/2 were inhibited in the Art + inhibitor group compared with the Art (25 µM) group. The results indicated that Art inhibited MEK/ERK and PI3K/Akt signaling pathways.
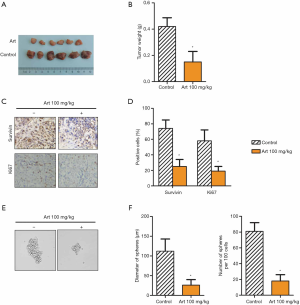
Art inhibited growth and stemness in KG1axenograft models
KG1a xenograft mouse models were established to confirm the anticancer role of Art in vivo. Some mice were treated with Art and some were not (100 mg/kg). Tumor size (Figure 5A) and tumor weight (Figure 5B) were significantly decreased by Art. Immunohistochemistry analysis (Figure 5C,D) showed that Art significantly reduced the expression of Ki67 and survivin, indicating that cell proliferation was restricted. Art also impeded the cell sphere formation ability as evidenced by reduced diameters of spheres and sphere numbers (Figure 5E,F) in vivo. These results suggest that Art inhibited growth and stemness in KG1a xenograft models.
Discussion
Due to advances in chemotherapy regimens and supportive therapy, the application of hematopoietic stem cell transplantation, and a greater understanding of its pathogenesis, the prognosis of leukemia has improved (14). However, many obstacles, such as multi-drug chemoresistance, high cost, severe side effects, and limited bone marrow bank resources, have greatly restricted the treatment of leukemia in modern medicine (15). Therefore, there has been an intensification of research into developing effective and low toxic anti-tumor cell agents from natural drugs.
Pharmacological studies have confirmed that artemisinin and its derivatives not only have antimalaria and antitumor effects but can also inhibit the proliferation of white blood cells (16). Pharmacokinetic studies have proven that artemisinin can be absorbed quickly, distributed widely, and excreted quickly from the body (17), while toxicological studies have confirmed that its side effects are mild (18). Interestingly, the main target organ of artemisinin toxicity is bone marrow (19). Artemisinin can inhibit bone marrow hematopoiesis and has a strong targeting effect on leukemia cells, which is one of the characteristics of antileukemia drugs. Art, as a derivative of artemisinin, has long been used clinically as an antimalarial drug (20). In vivo and in vitro experiments have shown that Art can selectively kill tumor cells and reverse the multidrug resistance of tumor cells, without cross-resistance to traditional chemotherapy drugs (21). Therefore, Art may serve as a new type of antileukemia drug in clinical application. Many researchers have studied the antitumor effect and mechanism of Art, and the main anti-tumor mechanisms primarily involve proliferation inhibition and apoptosis promotion.
Survivin is expressed in most human tumors, and can inhibit key members of the caspase protease family (such as caspase-3 and -7) and exert an apoptosis-inhibiting effect. P21, the first CKI gene found, mainly acts by modulating the activity of CDK. P21 blocks the activity of all cyclin-CDK complexes, such as cyclin E-CDK2, cyclin D-CDK4, and cyclin A-CDK2 (22). Overactivation of the P21 gene can result in cell cycle disorders and block cell proliferation (23). Previous studies have shown that, in conjunction with surviving, p21 and p27 may also inhibit cell apoptosis (24,25). Results of this study suggest that Art enhances tumor cells apoptosis and inhibits cell proliferation.
Increasing evidence shows that leukemia stem cells have an important role in chemoresistance and relapse of leukemia (26). In this study, we first explored the role of Art on stemness of leukemia cells, which showed that Art inhibited stemness of leukemia cells, as evidenced by confined sphere formation and reduced expressions of stem cell marker proteins including CD44, SOX2, ALDH1, and OCT4.
PI3K/Akt and MEK/ERK signaling pathways play important roles in mediating extracellular stimulation to intracellular response, regulating gene expression, and inhibiting apoptosis (27). Inhibition of Akt activity with PI3K inhibitor LY294002 or the protein synthesis inhibitor actinomycin can increase TRAIL-induced apoptosis, while overexpression of active Akt makes TRAIL-sensitive cell line SUN-668 resistant to induced apoptosis (28). The addition of MEK1/2 inhibitor in Hela cells also significantly increase the rate of apoptosis induced by TRAIL, indicating the protective effect of cells against apoptosis (29). Recent studies have shown that the MEK/ERK cascade phosphorylates Thr125 on caspase-9, thereby inactivating caspase-9 (30). In this study, PI3K/Akt and MEK/ERK signaling pathways were inhibited by Art. This result suggests that Art may promote apoptosis of leukemia cells via inactivating PI3K/Akt and MEK/ERK signaling pathways.
Cancer stem cells, which are defined by self-renewal, differentiation potential and tumorigenicity, are proposed to be responsible for cancer initiation and maintain the tumor mass. Typically, growth factors are known to stimulate MEK/ERK pathways, which is responsible for mitogenic activity, while the PI3K appears more related with the maintenance of stemness (31). The ING family of epigenetic regulators (ING5) also enhances PI3K/AKT and MEK/ERK activity to sustain self-renewal of Stem cell-like brain tumor initiating cells (BTICs) over serial passage of stem cell-like spheres (32). CD44 is an important oral cancer stem cell marker, CD44v4 expression is more linked with ERK1/2 activation and promote cisplatin resistance, whereas CD44v6 expression is associated primarily with PI3K/Akt/GSK3β activation and driving tumor invasion/migration (33). Bufalin inhibited the activation of MEK/ERK and PI3K/AKT signaling pathways by inhibiting the expression of p-c-Met, thus inhibiting proliferation of GBC-SD cells and reducing the self-renewal ability of gallbladder cancer stem cells (34). Activation of PI3K/Akt and MEK/ERK signaling pathways has been proven to promote stemness of cancer cells (35,36). Consistent with the previous studies, Art inhibited stemness of leukemia cells, and interestingly, this mechanism might be regulated by PI3K/Akt and MEK/ERK signaling pathways.
In conclusion, this study showed that Art inhibited proliferation and stemness of leukemia cells in vitro and in vivo, and promoted their apoptosis. The mechanism of Art regulation of cell apoptosis, proliferation, and stemness consist of the modulation of PI3K/Akt and MEK/ERK signaling pathways.
Acknowledgments
Funding: Funding is provided the First Affiliated Hospital of Zhengzhou University.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4558
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4558
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4558). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Each of the animal experiments in this study was conducted according to the principles of the NIH Guide for the Care and Use of Laboratory Animals and received approval from The First Affiliated Hospital of Zhengzhou University (No. 20190918).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 2012;30:1663-9. [Crossref] [PubMed]
- Zhang HH, Wang HS, Qian XW, et al. Genetic variants and clinical significance of pediatric acute lymphoblastic leukemia. Ann Transl Med 2019;7:296. [Crossref] [PubMed]
- Dondorp A, Nosten F, Stepniewska K, et al. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005;366:717-25. [Crossref] [PubMed]
- Wang Z, Wang C, Wu Z, et al. Artesunate Suppresses the Growth of Prostatic Cancer Cells through Inhibiting Androgen Receptor. Biol Pharm Bull 2017;40:479-85. [Crossref] [PubMed]
- Li Y, Feng L, Li Y, et al. Artesunate possesses anti-leukemia properties that can be enhanced by arsenic trioxide. Leuk Lymphoma 2014;55:1366-72. [Crossref] [PubMed]
- Kumar B, Kalvala A, Chu S, et al. Antileukemic activity and cellular effects of the antimalarial agent artesunate in acute myeloid leukemia. Leuk Res 2017;59:124-35. [Crossref] [PubMed]
- Tan M, Rong Y, Su Q, et al. Artesunate induces apoptosis via inhibition of STAT3 in THP-1 cells. Leuk Res 2017;62:98-103. [Crossref] [PubMed]
- Qin G, Wu L, Liu H, et al. Artesunate induces apoptosis via a ROS-independent and Bax-mediated intrinsic pathway in HepG2 cells. Exp Cell Res 2015;336:308-17. [Crossref] [PubMed]
- Wang Y, Yang J, Chen L, et al. Artesunate induces apoptosis through caspase-dependent and -independent mitochondrial pathways in human myelodysplastic syndrome SKM-1 cells. Chem Biol Interact 2014;219:28-36. [Crossref] [PubMed]
- Greenshields AL, Fernando W, Hoskin DW. The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells. Exp Mol Pathol 2019;107:10-22. [Crossref] [PubMed]
- Darwish NH, Sudha T, Godugu K, et al. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget 2016;7:57811-20. [Crossref] [PubMed]
- Hoffman LM, Hall L, Batten JL, et al. X-inactivation status varies in human embryonic stem cell lines. Stem Cells 2005;23:1468-78. [Crossref] [PubMed]
- Drenberg CD, Buaboonnam J, Orwick SJ, et al. Evaluation of artemisinins for the treatment of acute myeloid leukemia. Cancer Chemother Pharmacol 2016;77:1231-43. [Crossref] [PubMed]
- Jackson K, Kennedy G, Mollee P, et al. Intensive chemotherapy and reduced-intensity allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia in elderly patients. Asia Pac J Clin Oncol 2014;10:246-54. [Crossref] [PubMed]
- Frolova O, Samudio I, Benito JM, et al. Regulation of HIF-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther 2012;13:858-70. [Crossref] [PubMed]
- Du JH, Zhang HD, Ma ZJ, et al. Artesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xenografts in vivo. Cancer Chemother Pharmacol 2010;65:895-902. [Crossref] [PubMed]
- Li HJ, Wang W, Tao YH, et al. Dihydroartemisinin-praziquantel combinations and multiple doses of dihydroartemisinin in the treatment of Schistosoma japonicum in experimentally infected mice. Ann Trop Med Parasitol 2011;105:329-33. [Crossref] [PubMed]
- Rasheed A, Khan SM, Awan MY, et al. Efficacy and safety of artemehter-lumefantrine in uncomplicated falciparum malaria in Liberia. J Pak Med Assoc 2011;61:131-4. [PubMed]
- Rahaman M, Ghosh S, Chowdhury LD, et al. Evaluation of anti-leishmanial activity of artemisinin combined with amphotericin B or miltefosine in Leishmania donovani promastigotes. International Journal of Basic & Clinical Pharmacology 2014;3:644-8. [Crossref]
- Reiter C, Fröhlich T, Zeino M, et al. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur J Med Chem 2015;97:164-72. [Crossref] [PubMed]
- Efferth T, Giaisi M, Merling A, et al. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One 2007;2:e693. [Crossref] [PubMed]
- Li Y, Yu H, Han F, et al. Biochanin A Induces S Phase Arrest and Apoptosis in Lung Cancer Cells. Biomed Res Int 2018;2018:3545376.
- Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell 2002;2:103-12. [Crossref] [PubMed]
- Suzuki A, Ito T, Kawano H, et al. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene 2000;19:1346-53. [Crossref] [PubMed]
- Temme A, Diestelkoetter-Bachert P, Schmitz M, et al. Increased p21(ras) activity in human fibroblasts transduced with survivin enhances cell proliferation. Biochem Biophys Res Commun 2005;327:765-73. [Crossref] [PubMed]
- Styczynski J, Drewa T. Leukemic stem cells: from metabolic pathways and signaling to a new concept of drug resistance targeting. Acta Biochim Pol 2007;54:717-26. [Crossref] [PubMed]
- Steelman LS, Abrams SL, Whelan J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 2008;22:686-707. [Crossref] [PubMed]
- McCubrey JA, Steelman LS, Abrams SL, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia 2008;22:708-22. [Crossref] [PubMed]
- Schmidt JT, Mariconda L, Morillo F, et al. A role for the polarity complex and PI3 kinase in branch formation within retinotectal arbors of zebrafish. Dev Neurobiol 2014;74:591-601. [Crossref] [PubMed]
- Tabusa H, Brooks T, Massey AJ. Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol Cancer Res 2013;11:109-21. [Crossref] [PubMed]
- Hassan G, Du J, Afify SM, et al. Cancer stem cell generation by silenced MAPK enhancing PI3K/AKT signaling. Med Hypotheses 2020;141:109742. [Crossref] [PubMed]
- Wang F, Wang AY, Chesnelong C, et al. ING5 activity in self-renewal of glioblastoma stem cells via calcium and follicle stimulating hormone pathways. Oncogene 2018;37:286-301. [Crossref] [PubMed]
- Kashyap T, Pramanik KK, Nath N, et al. Crosstalk between Raf-MEK-ERK and PI3K-Akt-GSK3β signaling networks promotes chemoresistance, invasion/migration and stemness via expression of CD44 variants (v4 and v6) in oral cancer. Oral Oncol 2018;86:234-43. [Crossref] [PubMed]
- Qian L, Su H, Wang G, et al. Anti-tumor Activity of Bufalin by Inhibiting c-MET Mediated MEK/ERK and PI3K/AKT Signaling Pathways in Gallbladder Cancer. J Cancer 2020;11:3114-23. [Crossref] [PubMed]
- Wang L, Wu Q, Li Z, et al. Delta/notch-like epidermal growth factor-related receptor promotes stemness to facilitate breast cancer progression. Cell Signal 2019;63:109389. [Crossref] [PubMed]
- Wei F, Zhang T, Deng SC, et al. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett 2019;450:1-13. [Crossref] [PubMed]



