A review of the management of patients with advanced heart failure in the intensive care unit
Introduction
The definition of advanced heart failure (AHF) has evolved over the years. In 1998, Adams and Zannad limited the description to those patients with resting left ventricular ejection fraction (LVEF) <30%, and the presence of New York Heart Association (NYHA) class 3 or class 4 symptoms, or peak oxygen consumption <14 mLs/kg/min, on symptom limited exercise testing (1). With time, the definition has evolved (2-5); the contemporary statement is that from the Heart Failure Association of the European Society of Cardiology (ESC) (6). In addition to the aforementioned criteria this ESC definition recognizes patients in whom isolated right ventricular (RV) failure, inoperable valvular disease, congenital heart disease or heart failure with either preserved ejection fraction (HFpEF), or mid-range ejection fraction (HFmrEF) represents the underlying structural abnormality. These patients must also have had more than one unplanned hospital attendance or admission within the preceding 12 months for the treatment of congestion, low output state or malignant arrhythmia (6).
Despite the advances in medical and device therapy for heart failure (HF), the prognosis for patients with AHF remains poor. The Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management (ROADMAP) study enrolled 200 patients with non-inotrope dependent AHF across 41 centers in the United States and showed that survival was inferior at 12 months (80%±4% vs. 63%±5%; P=0.022), and 24 months (70%±5% vs. 41%±5%; P<0.001) in those managed in the medical therapy arm (7,8). Similarly, just over half of the patients with ambulatory AHF in the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry were alive on medical therapy alone at 2 years of follow-up, 24% died over the study period and 11% underwent left ventricular assist device (LVAD) implantation; 12% received CTx (9).
An episode of decompensation in the patient with AHF invariably results in hospitalization. Whilst many HF decompensations are managed at a ward-based level, a proportion of patients may require admission to the intensive care unit (ICU) for higher acuity care. The aims of this article are to discuss the management of patients with AHF in the ICU. The pre-operative optimization for those patients requiring mechanical circulatory support (MCS) or CTx are also discussed.
Acute heart failure (AcHF)
AcHF is the rapid development of, or worsening of symptoms and signs of HF typically leading to hospitalization (10). This may present in a patient with known HF or as a de-novo event. In the United Kingdom, the National Heart Failure Audit for 2016-17 reported that for England and Wales alone 73,616 patients were admitted to hospital with a primary diagnosis of HF (11). The median age of patients was 80.6 years, and the in-hospital mortality 9.4% after a 9-day median length of stay (11). For several reasons, there is wide center specific, and international variation in the admission rates to ICU for HF patients. The UK National Heart Failure audit does not detail the percentage of patients who are admitted to ICU. Across Europe alone admission to ICU varies from 5% to 45.4% (12). The prospective Romanian Acute Heart Failure Syndromes (RO-AHFS) registry found that 10.7% of 3,224 patients required care within the ICU, and that admission to ICU was associated with a higher risk of in-hospital mortality (17.3% vs. 6.5%, P=0.002) (13).
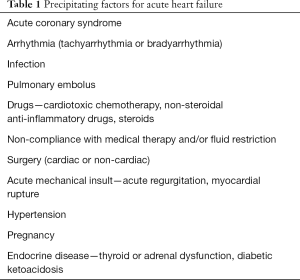
There are many potential precipitants to an episode of decompensated HF. The most commonly encountered triggers in clinical practice are listed in Table 1.
Full table
Clinical assessment of patients with AcHF
Hemodynamic profiles and prognosis
Advanced HF patients admitted to the ICU are usually in AcHF. Irrespective of whether the presentation is a decompensation of chronic HF or first presentation, this is a life-threatening scenario with very high mortality. Thirty-day mortality ranges from 12.9% to 27.4% and 1 year mortality from 39.7–46.5% (14,15). In hospital worsening of HF requiring advanced therapies (including inotropic or intravenous vasodilator therapy, MCS, mechanical ventilation or hemodialysis) is associated with an even higher mortality rate; 12.7% in hospital, 19% 30 days and 50% 1 year mortality (15).
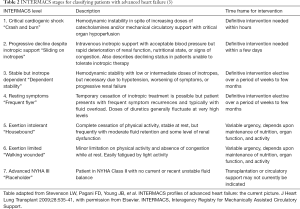
Classification for this group of patients can be performed in several ways. The Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) classification (Table 2) stratifies inpatients and outpatients with AHF who are awaiting durable MCS into one of seven profiles (3). A simple and alternative classification based on the assessment of congestion and peripheral perfusion can be used for inpatients with AcHF. This defines four different hemodynamic profiles; warm and wet, cold and wet, warm and dry, and cold and dry (16). Ninety five percent of AcHF patients are congested (17). Recognizing that hypo perfusion does not automatically equate to hypotension is important. Cold extremities, oliguria, confusion, narrow pulse pressure, metabolic acidosis and elevated serum lactate can be signs of impaired peripheral perfusion even with preserved blood pressure. Therefore, identifying the correct clinical profile helps guide therapy and prognosis (16).
Monitoring of hemodynamics on ICU
There is no universal agreement on the optimal method of hemodynamic monitoring for patients admitted to ICU with AcHF. The current American College of Cardiology/American Heart Association (ACC/AHA) guidelines give a class I recommendation for monitoring with a pulmonary artery catheter (PAC) in AcHF patients with respiratory distress or impaired systemic perfusion, when clinical assessment is inadequate (4). The current ESC guidelines recommend consideration of an intra-arterial line and PAC in patients with hypotension and hypo perfusion despite treatment (17).
PAC monitoring is used in approximately a third of cardiogenic shock patients in European tertiary level hospitals (18). There are no randomized trials evaluating the use of PAC in the MCS population. In the ICU setting, randomized clinical trials have failed to demonstrate an improvement in clinical outcomes with the use of PACs. The sickest AcHF patients are under-represented in these studies however (19,20). The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial studied the use of PAC specifically in an AcHF population and showed no mortality benefit with PAC use. These results should be interpreted with caution however as the study excluded those with previous inotrope use and significant renal impairment (21). In a propensity matched analysis of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry (a cohort of 4,842 patients with acute decompensated heart failure in Japan), the appropriate use of PAC (502 patients matched to controls) reduced in-hospital mortality in AcHF (4.4% controls vs. 1.4% in the PAC groups, P=0.006), particularly in patients with lower systolic blood pressure, or receiving inotropic support (22).
PACs provide an accurate assessment of filling pressures and pulmonary hemodynamics which are essential when optimizing patients prior to LVAD surgery and CTx. Cardiac Index and its derived parameters cardiac power index and stroke volume index are strong predictors of 30-day mortality in patients with cardiogenic shock (18). Obtaining and interpreting these data help when assessing the deteriorating patient, and identifying those who may be suitable for MCS or transplantation.
Treatment in the ICU
Management goals
Decongestion, maintenance of adequate systemic perfusion and preservation of end organ function are the primary goals of treatment of AcHF patients. The combination of pharmacological and non-pharmacological interventions is often required to achieve these. These strategies may be a temporizing measure, a bridge to recovery or a definitive treatment option such as durable MCS or CTx. Optimizing clinical status prior to surgery is imperative, as critically ill patients have poorer outcomes following CTx and LVAD surgery (23-26).
Supplemental oxygen and ventilation
Oxygen therapy is recommended only in patients with SpO2 <90% or PaO2 <60 mmHg (9.0 kPa). Noninvasive ventilation should be considered in patients with acute pulmonary edema to relieve symptoms and reduce the need for intubation. In a small number of studies, it has been shown to reduce mortality (27-29). Noninvasive ventilation can exacerbate hypotension and should be used with caution in patients with low or borderline blood pressure. Intubation may be required for patients with persistent hypoxemia, hypercapnia or acidosis.
Management of congestion
Elevated central venous pressure (CVP) is the most important hemodynamic factor in the development of worsening renal function and unfavourable outcomes in AcHF patients (30). Decongestion and the maintenance of normovolemia can be challenging, and often requires a progressive escalation of therapy.
Diuretics
Intravenous loop diuretics should be administered to all patients with congestion. Diuretics can be given as a continuous infusion or intermittent boluses with the initial dose at least equal to the pre-existing oral dose (31). Data from a meta-analysis has shown that a continuous infusion of loop diuretics is superior to intermittent boluses with regards to diuretic effect, with no impact on mortality (32). For diuretic resistance, the addition of metolazone, intravenous chlorothiazide or tolvaptan have all been shown to improve urine output without significant difference between the agents (33). Electrolytes and renal function must be closely monitored, particularly with the addition of sequential nephron blockade.
Ultrafiltration and renal replacement therapy
The development of diuretic resistance portends poorer long-term outcomes in hospitalized patients with AcHF (34). The major clinical trials evaluating ultrafiltration versus intravenous diuretics have reported inconsistent results (35-37); however, ultrafiltration does appear to have a role for management of refractory congestion not responding to medical therapy. Its use in this situation is supported by both ESC (17) and ACC/AHA guidelines (4). Ultrafiltration has the benefit of being able to remove large volumes of fluid in a relatively short period of time and can be particularly beneficial in optimizing patients prior to definitive surgery. Renal replacement therapy may also be required for patients with refractory congestion and acute kidney injury, particularly when accompanied by hyperkalemia or metabolic acidosis.
Vasodilators and inotropes
Vasodilators and inotropes have a similar impact on the reduction of left- and right-sided filling pressures in patients with AcHF and reduced LV function (38). Vasodilators reduce preload which relieves congestion and are also used to decrease afterload, which can help to increase cardiac output. They improve hemodynamics in the short-term. There is no evidence of a mortality benefit from vasodilator use (38-42) and their utility is limited to patients with adequate blood pressure (generally systolic blood pressure >90 mmHg). Nitrates are the most common vasodilator used in AcHF. Serelaxin and Ularitide are newer vasodilators; however, these have also been shown to have no significant benefit on long term outcomes in recent randomized control trials (40,42). Table 3 lists the properties of commonly used vasodilators.

Full table
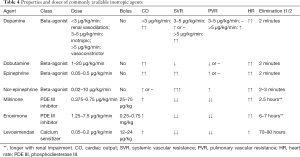
Patients with low cardiac output and end-organ hypoperfusion may be treated with inotropes. Although the published evidence suggests that treatment with inotropes is associated with an increased short- and long-term mortality (43), they may be used to bridge patients with AcHF to recovery, or definitive treatment. Inotropic agents should be used at the lowest required dose, for the shortest duration possible. There is no evidence to support the superiority of one agent over another (44). There are three main classes of inotropes currently used in the management of AcHF, beta adrenergic receptor agonists, phosphodiesterase III (PDE III) inhibitors and calcium sensitizer. Table 4 displays the properties of commonly used inotropes. Patients treated with beta blockers (BB) may respond better to levosimendan or PDE III inhibitors, as these drugs act independently of the beta-adrenergic receptor pathway (45,46).
Full table
Optimizing patients prior to MCS, or cardiac transplantation
Kidneys
Over half of the 118,465 patients hospitalized with AcHF in the Acute Decompensated Heart Failure National Registry (ADHERE) in the United States had renal dysfunction (eGFR ≤59 mL/min/1.73 m2), with in hospital mortality increasing from 1.9% in those with normal renal function to 7.6% in those with severe renal dysfunction (eGFR 15–29 mL/min/1.73 m2) (47). In a study of 599 patients admitted to 60 French ICUs or coronary care units for the management of AcHF, renal dysfunction was associated with a greater than three-fold increased risk of death at 4 weeks (14). The presence of renal dysfunction is also associated with adverse outcomes following LVAD implantation with an almost 20% reduction in 2-year survival going from low to severe dysfunction in an analysis from the INTERMACS registry (48).
The timing of hemodialysis in those undergoing LVAD implantation has an impact on short-term survival. Schmack et al. reported significantly worse 30-day survival after LVAD implantation in those requiring post implant hemodialysis (92.1% in non-hemodialysis group; 83.3% in pre-implant dialysis group; 58.3% in post implant hemodialysis group; P<0.004) (49). In a study of 389 patients undergoing implantation of continuous flow LVAD, eGFR <40 mL/min/1.73 m2 and proteinuria (urine protein to creatinine ratio ≥0.55 mg/mg) were significant predictors of the requirement for renal replacement therapy during a median follow-up of 9.9 months (50). An eGFR <30 mL/min/1.73 m2 is a relative contraindication for heart transplantation alone, according to the 2016 ISHLT listing criteria for heart transplantation (51).
In HF patients with renal dysfunction, a right heart catheter study (RHC) to assess filling pressures and hemodynamics, urine analysis for proteinuria, and assessment of the kidneys and urinary tract on ultrasound or CT should be performed. Longitudinal assessment of the trajectory of renal function is intuitively attractive when considering the potential for improvement in renal function following optimization of hemodynamics. In those with significant renal impairment being considered for LVAD or cardiac transplantation, our institution would recommend the cessation of nephrotoxic drugs, minimization of exposure to iodinated contrast and optimization of adverse hemodynamics, with judicious use of diuretics, escalation of inotropes and consideration of temporary MCS.
Liver
Congestive hepatopathy is more common than reduced cardiac output as a cause of liver dysfunction in patients with HF (52). In the analysis of the 2679 North American patients enrolled in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity Program (CHARM); low albumin (18.3%), raised total bilirubin (13%) and increased alkaline phosphatase (14%) were the most common liver function abnormalities (53). Elevated bilirubin was the strongest independent predictor of poor prognosis in this cohort. More than 40% of the 4,228 patients hospitalized with HF in the Acute Study of Clinical Effectiveness of Neseretide in Decompensated Heart Failure (ASCEND-HF) trial had abnormal liver function (54). This study also showed that elevated serum bilirubin was associated with an increased risk (HR 1.17 per 1 mg/dL increase, 95% CI, 1.04–1.32, P=0.13) of 30-day all cause death, or HF re-hospitalization. No relationship was found between aminotransferases and outcomes. Hypoxic or ischemic hepatitis is due to a combination of low cardiac output and passive hepatic venous congestion (55). There tends to a rapid rise in serum aminotransferases peaking at 1–3 days (up to 250 times the upper limit of normal) after a hemodynamic insult (52). Bilirubin levels rarely rise more than 4 times above the upper limit of normal, and alkaline phosphatase is usually within 2 times the upper limit of normal. Levels generally return to baseline within 7–10 days with supportive treatment (52).
Coagulation disorders, vasoplegia, immune dysfunction and poor nutritional status are associated with advanced liver disease (56). The Model for End-Stage Liver Disease (MELD) score is an objective score based on bilirubin, creatinine and INR. The MELD-XI (MELD eXcluding INR) score excludes INR and is an alternative to MELD in those receiving oral coumadins. A single center study of 264 patients undergoing LVAD implantation (60% pulsatile HeartMate and 40% continuous flow HeartMate II) reported improved on-VAD and overall survival in patients with a MELD score or MELD-XI <17 (57). A more recent study of 524 patients implanted with continuous flow LVADs (403 Heart Mate II, 123 HeartWare) demonstrated lower survival at 1, 3, 6, 12 and 24 months (P<0.001 for all) and increased risk of early right heart failure and infections in those with MELD-XI score of 14 or more, compared to patients with a MELD-XI score of less than 14 (58).
Elevated MELD scores are also associated with poor survival following heart transplantation. In a study of 617 adults undergoing heart transplantation, patients with MELD score <14 had 1- and 5-year survival of 91.4% and 83.2% respectively. One- and five-year survival rates were 85.5% and 70.1% respectively in those with MELD score >20 (59). These scores are dynamic and can change with progressive HF, or improve with therapy. In patients with imaging features suggestive of fibrosis or cirrhosis of the liver, the addition of liver biopsy to MELD-XI improves risk stratification in patients with advanced HF being considered for heart transplantation (60).
In patients with AHF being considered for MCS or heart transplantation serial liver function tests (LFTs) should be performed. Patients with persistently deranged LFTs despite restoration of normovolemia and improvement in cardiac output following treatment should undergo further assessment with dedicated liver imaging. The specialist opinion of a hepatologist should be sought to exclude irreversible liver disease, or an alternative cause for deranged liver function.
Anemia and coagulation
The prevalence of anemia in patients with HF is reported between 6–70% reflecting the heterogeneity in screening, clinical setting and socio-economic status of the population (61). Pre-operative anemia is a known risk factor for adverse outcomes following cardiac surgery (62-64). Pre-transplant anemia is also an independent predictor of 1-year survival. In a single center study of 267 patients, one year survival was 70% among anemic patients (serum hemoglobin <12 g/dL regardless of sex) compared with 81% for those with a normal hemoglobin (P=0.027) (65). The Society of Thoracic Surgeons (STS) guidelines recommend transfusion when hemoglobin is less than 7 g/dL but acknowledge the lack of high level evidence to support this recommendation (66).
Iron deficiency (defined as serum ferritin <100 μg/L or ferritin <300 μg/L and transferrin saturation level of <20%) is seen in up to 50% of patients with HF and is an independent predictor of poor exercise capacity and worse survival (67,68). There is strong evidence to recommend the use of intravenous iron in iron deficient HF patients to improve exercise capacity and symptoms (10,69). Its recommendation (with or without erythropoietin) in anemic patients undergoing cardiac surgery is largely unproven (70,71).
HF is a hypercoagulable state (72). Patients with co-morbidities such as ischemic heart disease, atrial fibrillation, previous thromboembolic events, pulmonary emboli or deep vein thrombosis are likely to be on anti-platelets and/or anticoagulated. The STS guidelines recommend cessation of platelet P2Y12 receptor blockers at least 3 days before cardiac surgery (66). It also recommends consideration of discontinuation of most antithrombotic agents before surgery to reduce minor and major bleeding events, with the timing of discontinuation based on drug half-life and availability of reversal agents. Unfractionated heparin may not require discontinuation (66).
In our center, patients being considered for durable LVAD or listing for CTx intravenous iron is administered to those with iron deficiency. Platelet P2Y12 receptor antagonists are stopped, if possible, 3–5 days prior to elective LVAD implantation. Patients on direct oral anticoagulants (DOACs) are switched to warfarin prior to transplant listing. DOACs are stopped in those awaiting elective LVAD, with heparin bridging if required. In patients with liver dysfunction and deranged clotting, vitamin K is administered.
Glycemic control
Patients with HF and diabetes mellitus (DM) have a greater risk of recurrent hospitalization and death compared to non-diabetics (73). In patients undergoing cardiac surgery diabetes is an independent predictor for post-operative sternal instability with or without infection, perioperative stroke, post-operative delirium, prolonged ICU stay and renal dysfunction (74). In patients with DM undergoing LVAD implantation, there is insufficient data to determine whether short term glycemic control prior to LVAD implantation is related to the risks of device complications and mortality (75). The STS guidelines recommend cessation of oral hypoglycemic drugs 24 hours prior to scheduled cardiac surgery (76). The guidelines also recommend stopping insulin after dinner the evening before surgery and using either an insulin infusion protocol or combination of long and short acting subcutaneous insulin.
Nutrition and sarcopenia
Malnutrition and sarcopenia is common in patients with HF. Up to 15% are overtly cachectic, 50% malnourished using broader definitions (77) and 20% sarcopenic compared with age matched controls (78). Patients at extremes of body mass index (<20 kg/m2; >35 kg/m2) have worse outcomes after LVAD implantation (79). The 2013 ISHLT guidelines recommend measuring serum albumin and pre-albumin prior to surgery (80). Those with indices of malnutrition should be evaluated by nutritional services. Resistance training is the main intervention used to improve sarcopenia and in combination with aerobic work is recommended both before and after transplantation (81). The use of testosterone supplementation is controversial (82).
Cardiac drugs
Patients with AcHF or cardiogenic shock in ICU are usually treated with inotropes for hemodynamic support. Levosimendan is a calcium channel sensitizer and an inotrope with vasodilatory effects. It provides a sustained hemodynamic response (for days after discontinuation of infusion) in patients with left ventricular impairment, without an increase in myocardial oxygen demand or ischemia (83). Meta-analyses comparing levosimendan and conventional treatment in cardiac surgical patients with left ventricular impairment report a reduction in perioperative mortality and need for renal replacement therapy (84,85). There remains insufficient high-quality evidence however to support or contraindicate its use (85). There may be a role for levosimendan as a pre-treatment for LVAD patients however the evidence to support this is not conclusive (86-88). A single center study of 21 patients reported an improvement in hemodynamics following levosimendan but no impact on reduction in post-operative RV failure (89).
Patients with chronic HF are usually on a combination of medical therapy including angiotensin converting enzyme inhibitor (ACEi) or angiotensin 2 receptor blockers (ARB), BB, mineralocorticoid receptor antagonists (MRA) and diuretic. Sacubitril/valsartan may be substituted in place of ACEi or ARB (10). In those with decompensated HF on ICU some of these drugs may have been stopped on admission because of hypotension, hypoperfusion and/or renal impairment. The use of an ACEi/ARB pre LVAD implant has been shown to have a negative correlation with improvement in glomerular filtration rate at one month post implant (90). Similarly, ACEI/ARB use has been reported to be a predictor of the requirement for early post-transplant renal replacement therapy (91). Profound vasoplegia in the post-transplant period has been described in a patient taking sacubitril/valsartan (92), and this drug should be discontinued on admission to ICU.
There is lack of data on the impact of pre-implant BB on post LVAD or post-transplant outcomes. In our institution, BB may be continued if the patient was pretreated and tolerating these. We do not recommend the initiation of BB in those with AcHF or cardiogenic shock prior to the restoration of normovolemia and adequate perfusion.
Decolonization
The prevention of post-surgical site infection (SSI) begins pre-operatively. Decolonization with topical chlorhexidine, and nares with mupirocin may reduce the risk of SSI (93,94). There is insufficient data at present to recommend its routine use in patients undergoing MCS, and practice varies by individual surgical center.
Specific issues
Patients undergoing implantation of temporary circulatory support
The placement of temporary MCS is often performed on an emergent basis. The device strategy and necessary work-up and are discussed separately within this issue of the journal. An assessment of the peripheral vasculature (femoral, lilac and thoraco-abdominal aorta) on CT provides additional information to inform device strategy, should this be a semi-planned procedure.
Patients undergoing durable LVAD implantation
An LVAD is dependent upon a functional RV for adequate filling. The RV functions to maintain a low systemic venous pressure, provide pulmonary circulation and fill the left ventricle (LV). All walls of the RV contribute to its function and the septum is a significant contributor in the situation of increased RV afterload (95). The implantation of an LVAD reduces RV afterload by reducing pulmonary pressure however this also increases RV pre-load. The septal contribution to RV contractility is impacted by leftward deviation of the interventricular septum, and the surgical disruption of the pericardium leads to weakening of the LV/RV interplay.
The evaluation of RV function in the ICU is performed through a combination of imaging and hemodynamic assessment. Several studies have examined the utility of RV assessment on echocardiography in predicting post LVAD RV failure however these series are small and limited due to the complex RV geometry, adequate RV visualization and the impact of loading conditions. Semi quantitative assessment of RV function has poor reproducibility. Tricuspid annular plane systolic excursion (TAPSE) <7.5 mm is specific in predicting post LVAD RV failure, with poor sensitivity (96). Severe tricuspid regurgitation (grade 3/4) is also predictive of post LVAD RV failure (97).
Multiple hemodynamic abnormalities (Table 5) have been shown to be predictive of post implant RV failure (98-101); however, these cannot account for intra-operative events which may insult a previously adequate RV. Pre-operative RV optimization includes strategies which aim to lower CVP, and decrease pulmonary artery pressures (PAP). A pre-operative CVP >15 mmHg is associated with RV failure (98). We generally aim for a CVP <10 mmHg through a combination of diuresis or filtration. PDE III inhibitors provide inotropic support and vasodilatation, and may be more useful than catecholaminergic agents (102).

Full table
Cardiac transplantation
Cardiac transplantation is a treatment option for a few carefully selected patients with AHF (103). In the ICU, patients waiting for CTx are those requiring multiple inotropes or patients on short term MCS. Multiple transfusions can increase the risk of allosensitization and impact upon the ability to match an organ (104). Minimizing blood transfusion in patients waiting for CTx is essential. Those requiring blood should receive leucodepleted blood, but do not require cytomegalovirus negative blood (105). Higher rates of primary graft dysfunction have been reported in recipients with right atrial pressure >10 mmHg and in those with elevated pulmonary vascular resistance (106). We recommend measuring CVP and PA pressures in inotrope dependent patients awaiting CTx aiming for CVP <10 mmHg and PVR <5 Wood units.
Conclusions
The prognosis for patients with AcHF requiring admission to ICU is poor and hemodynamic profiling is important in guiding management. These critically ill patients benefit from the care of a multi-disciplinary team comprising the cardiac intensivist/anesthetist, HF cardiologist, cardiac surgeon and allied health professionals. Treatment goals are agreed upon by this team, and the ICU care includes optimization of volume status, vasodilatation and use of inotropes as a bridge to recovery of end organ function. In those suitable for MCS or CTx, a PAC is essential in hemodynamic tailoring prior to surgery. Finally, the timing of surgery, if required, is planned once these objectives are met in order to maximize the possibility of a successful outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kamen Valchanov) for the series “Perioperative Management of Patients with undergoing Mechanical Circulatory Support” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1048). The series “Perioperative Management of Patients with undergoing Mechanical Circulatory Support” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adams KF Jr, Zannad F. Clinical definition and epidemiology of advanced heart failure. Am Heart J 1998;135:S204-15. [Crossref] [PubMed]
- Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007;9:684-94. [Crossref] [PubMed]
- Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant 2009;28:535-41. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [Crossref] [PubMed]
- Fang JC, Ewald GA, Allen LA, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail 2015;21:519-34. [Crossref] [PubMed]
- Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1505-35. [Crossref] [PubMed]
- Estep JD, Starling RC, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol 2015;66:1747-61. [Crossref] [PubMed]
- Starling RC, Estep JD, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. JACC Heart Fail 2017;5:518-27. [Crossref] [PubMed]
- Ambardekar AV, Kittleson MM, Palardy M, et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant 2019;38:408-17. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Outcomes NIfCR. National Heart Failure Audit 2016/17 Summary Report. 2018. Available online: https://www.nicor.org.uk/wp-content/uploads/2018/11/Heart-Failure-Summary-Report-2016-17.pdf. Accessed 11/01/2020 2020.
- Chioncel O, Mebazaa A. Characteristics of Intensive Care in Patients Hospitalized for Heart Failure in Europe. Heart Fail Clin 2015;11:647-56. [Crossref] [PubMed]
- Chioncel O, Ambrosy AP, Filipescu D, et al. Patterns of intensive care unit admissions in patients hospitalized for heart failure: insights from the RO-AHFS registry. J Cardiovasc Med (Hagerstown) 2015;16:331-40. [Crossref] [PubMed]
- Zannad F, Mebazaa A, Juilliere Y, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail 2006;8:697-705. [Crossref] [PubMed]
- DeVore AD, Hammill BG, Sharma PP, et al. In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc 2014;3:e001088. [Crossref] [PubMed]
- Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41:1797-804. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Sionis A, Rivas-Lasarte M, Mebazaa A, et al. Current Use and Impact on 30-Day Mortality of Pulmonary Artery Catheter in Cardiogenic Shock Patients: Results From the CardShock Study. J Intensive Care Med 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 2005;366:472-7. [Crossref] [PubMed]
- Rhodes A, Cusack RJ, Newman PJ, et al. A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med 2002;28:256-64. [Crossref] [PubMed]
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005;294:1625-33. [Crossref] [PubMed]
- Sotomi Y, Sato N, Kajimoto K, et al. Impact of pulmonary artery catheter on outcome in patients with acute heart failure syndromes with hypotension or receiving inotropes: from the ATTEND Registry. Int J Cardiol 2014;172:165-72. [Crossref] [PubMed]
- Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant 2011;30:402-7. [Crossref] [PubMed]
- Alba AC, Rao V, Ivanov J, et al. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant 2009;28:827-33. [Crossref] [PubMed]
- Yoshioka D, Sakaguchi T, Saito S, et al. Predictor of early mortality for severe heart failure patients with left ventricular assist device implantation: significance of INTERMACS level and renal function. Circ J 2012;76:1631-8. [Crossref] [PubMed]
- Barge-Caballero E, Segovia-Cubero J, Almenar-Bonet L, et al. Preoperative INTERMACS profiles determine postoperative outcomes in critically ill patients undergoing emergency heart transplantation: analysis of the Spanish National Heart Transplant Registry. Circ Heart Fail 2013;6:763-72. [Crossref] [PubMed]
- Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med 2010;152:590-600. [Crossref] [PubMed]
- Gray A, Goodacre S, Newby DE, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008;359:142-51. [Crossref] [PubMed]
- Masip J, Roque M, Sanchez B, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 2005;294:3124-30. [Crossref] [PubMed]
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589-96. [Crossref] [PubMed]
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797-805. [Crossref] [PubMed]
- Ng KT, Yap JLL. Continuous infusion vs. intermittent bolus injection of furosemide in acute decompensated heart failure: systematic review and meta-analysis of randomised controlled trials. Anaesthesia 2018;73:238-47. [Crossref] [PubMed]
- Cox ZL, Hung R, Lenihan DJ, et al. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC Heart Fail 2020;8:157-68. [Crossref] [PubMed]
- Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261-70. [Crossref] [PubMed]
- Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296-304. [Crossref] [PubMed]
- Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675-83. [Crossref] [PubMed]
- Costanzo MR, Saltzberg MT, Jessup M, et al. Ultrafiltration is associated with fewer rehospitalizations than continuous diuretic infusion in patients with decompensated heart failure: results from UNLOAD. J Card Fail 2010;16:277-84. [Crossref] [PubMed]
- Ishihara S, Gayat E, Sato N, et al. Similar hemodynamic decongestion with vasodilators and inotropes: systematic review, meta-analysis, and meta-regression of 35 studies on acute heart failure. Clin Res Cardiol 2016;105:971-80. [Crossref] [PubMed]
- O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32-43. [Crossref] [PubMed]
- Metra M, Teerlink JR, Cotter G, et al. Effects of Serelaxin in Patients with Acute Heart Failure. N Engl J Med 2019;381:716-26. [Crossref] [PubMed]
- Teerlink JR, Davison BA, Cotter G, et al. Effects of serelaxin in patients admitted for acute heart failure: a meta-analysis. Eur J Heart Fail 2020;22:315-29. [Crossref] [PubMed]
- Packer M, O'Connor C, McMurray JJV, et al. Effect of Ularitide on Cardiovascular Mortality in Acute Heart Failure. N Engl J Med 2017;376:1956-64. [Crossref] [PubMed]
- Rossinen J, Harjola VP, Siirila-Waris K, et al. The use of more than one inotrope in acute heart failure is associated with increased mortality: a multi-centre observational study. Acute Card Care 2008;10:209-13. [Crossref] [PubMed]
- Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007;297:1883-91. [Crossref] [PubMed]
- Mebazaa A, Nieminen MS, Filippatos GS, et al. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur J Heart Fail 2009;11:304-11. [Crossref] [PubMed]
- Metra M, Nodari S, D'Aloia A, et al. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol 2002;40:1248-58. [Crossref] [PubMed]
- Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13:422-30. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J Heart Lung Transplant 2013;32:1205-13. [Crossref] [PubMed]
- Schmack B, Grossekettler L, Weymann A, et al. Prognostic relevance of hemodialysis for short-term survival in patients after LVAD implantation. Sci Rep 2018;8:8546. [Crossref] [PubMed]
- Topkara VK, Coromilas EJ, Garan AR, et al. Preoperative Proteinuria and Reduced Glomerular Filtration Rate Predicts Renal Replacement Therapy in Patients Supported With Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail 2016;9:e002897. [Crossref] [PubMed]
- Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant 2016;35:1-23. [Crossref] [PubMed]
- Sundaram V, Fang JC. Gastrointestinal and Liver Issues in Heart Failure. Circulation 2016;133:1696-703. [Crossref] [PubMed]
- Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 2009;11:170-7. [Crossref] [PubMed]
- Samsky MD, Dunning A, DeVore AD, et al. Liver function tests in patients with acute heart failure and associated outcomes: insights from ASCEND-HF. Eur J Heart Fail 2016;18:424-32. [Crossref] [PubMed]
- Henrion J, Descamps O, Luwaert R, et al. Hypoxic hepatitis in patients with cardiac failure: incidence in a coronary care unit and measurement of hepatic blood flow. J Hepatol 1994;21:696-703. [Crossref] [PubMed]
- Lopez-Delgado JC, Esteve F, Javierre C, et al. Influence of cirrhosis in cardiac surgery outcomes. World J Hepatol 2015;7:753-60. [Crossref] [PubMed]
- Yang JA, Kato TS, Shulman BP, et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant 2012;31:601-10. [Crossref] [PubMed]
- Critsinelis A, Kurihara C, Volkovicher N, et al. Model of End-Stage Liver Disease-eXcluding International Normalized Ratio (MELD-XI) Scoring System to Predict Outcomes in Patients Who Undergo Left Ventricular Assist Device Implantation. Ann Thorac Surg 2018;106:513-9. [Crossref] [PubMed]
- Chokshi A, Cheema FH, Schaefle KJ, et al. Hepatic dysfunction and survival after orthotopic heart transplantation: application of the MELD scoring system for outcome prediction. J Heart Lung Transplant 2012;31:591-600. [Crossref] [PubMed]
- Farr M, Mitchell J, Lippel M, et al. Combination of liver biopsy with MELD-XI scores for post-transplant outcome prediction in patients with advanced heart failure and suspected liver dysfunction. J Heart Lung Transplant 2015;34:873-82. [Crossref] [PubMed]
- Tim Goodnough L, Comin-Colet J, Leal-Noval S, et al. Management of anemia in patients with congestive heart failure. Am J Hematol 2017;92:88-93. [Crossref] [PubMed]
- Karkouti K, Wijeysundera DN, Beattie WS, et al. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 2008;117:478-84. [Crossref] [PubMed]
- Zindrou D, Taylor KM, Bagger JP. Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet 2002;359:1747-8. [Crossref] [PubMed]
- Cladellas M, Bruguera J, Comin J, et al. Is pre-operative anaemia a risk marker for in-hospital mortality and morbidity after valve replacement? Eur Heart J 2006;27:1093-9. [Crossref] [PubMed]
- Taegtmeyer AB, Rogers P, Breen JB, et al. The effects of pre- and post-transplant anemia on 1-year survival after cardiac transplantation. J Heart Lung Transplant 2008;27:394-9. [Crossref] [PubMed]
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Ferraris VA, Brown JR, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944-82. [Crossref] [PubMed]
- Cohen-Solal A, Leclercq C, Deray G, et al. Iron deficiency: an emerging therapeutic target in heart failure. Heart 2014;100:1414-20. [Crossref] [PubMed]
- von Haehling S, Ebner N, Evertz R, et al. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail 2019;7:36-46. [Crossref] [PubMed]
- Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436-48. [Crossref] [PubMed]
- Lim J, Miles L, Litton E. Intravenous Iron Therapy in Patients Undergoing Cardiovascular Surgery: A Narrative Review. J Cardiothorac Vasc Anesth 2018;32:1439-51. [Crossref] [PubMed]
- Hogan M, Klein AA, Richards T. The impact of anaemia and intravenous iron replacement therapy on outcomes in cardiac surgery. Eur J Cardiothorac Surg 2015;47:218-26. [Crossref] [PubMed]
- Gurbel PA, Tantry US. Antiplatelet and anticoagulant agents in heart failure: current status and future perspectives. JACC Heart Fail 2014;2:1-14. [Crossref] [PubMed]
- Gustafsson I, Brendorp B, Seibaek M, et al. Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. J Am Coll Cardiol 2004;43:771-7. [Crossref] [PubMed]
- Bucerius J, Gummert JF, Walther T, et al. Impact of diabetes mellitus on cardiac surgery outcome. Thorac Cardiovasc Surg 2003;51:11-6. [Crossref] [PubMed]
- Asleh R, Briasoulis A, Schettle SD, et al. Impact of Diabetes Mellitus on Outcomes in Patients Supported With Left Ventricular Assist Devices: A Single Institutional 9-Year Experience. Circ Heart Fail 2017;10:e004213. [Crossref] [PubMed]
- Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009;87:663-9. [Crossref] [PubMed]
- Rahman A, Jafry S, Jeejeebhoy K, et al. Malnutrition and Cachexia in Heart Failure. JPEN J Parenter Enteral Nutr 2016;40:475-86. [Crossref] [PubMed]
- Fulster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J 2013;34:512-9. [Crossref] [PubMed]
- Musci M, Loforte A, Potapov EV, et al. Body mass index and outcome after ventricular assist device placement. Ann Thorac Surg 2008;86:1236-42. [Crossref] [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [Crossref] [PubMed]
- Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003;107:1210-25. [Crossref] [PubMed]
- Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail 2018;5:1099-107. [PubMed]
- Harjola VP, Giannakoulas G, von Lewinski D, et al. Use of levosimendan in acute heart failure. Eur Heart J Suppl 2018;20:I2-I10. [Crossref] [PubMed]
- Sanfilippo F, Knight JB, Scolletta S, et al. Levosimendan for patients with severely reduced left ventricular systolic function and/or low cardiac output syndrome undergoing cardiac surgery: a systematic review and meta-analysis. Crit Care 2017;21:252. [Crossref] [PubMed]
- Xing Z, Tang L, Chen P, et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery: a meta-analysis and trial sequential analysis of randomized trials. Sci Rep 2018;8:7775. [Crossref] [PubMed]
- Theiss HD, Grabmaier U, Kreissl N, et al. Preconditioning with levosimendan before implantation of left ventricular assist devices. Artif Organs 2014;38:231-4. [Crossref] [PubMed]
- Santillo E, Migale M, Massini C, et al. Levosimendan for Perioperative Cardioprotection: Myth or Reality? Curr Cardiol Rev 2018;14:142-52. [Crossref] [PubMed]
- Landoni G, Biondi-Zoccai G, Greco M, et al. Effects of levosimendan on mortality and hospitalization. A meta-analysis of randomized controlled studies. Crit Care Med 2012;40:634-46. [Crossref] [PubMed]
- Sponga S, Ivanitskaia E, Potapov E, et al. Preoperative treatment with levosimendan in candidates for mechanical circulatory support. ASAIO J 2012;58:6-11. [Crossref] [PubMed]
- Hasin T, Topilsky Y, Schirger JA, et al. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol 2012;59:26-36. [Crossref] [PubMed]
- Jokinen JJ, Tikkanen J, Kukkonen S, et al. Natural course and risk factors for impaired renal function during the first year after heart transplantation. J Heart Lung Transplant 2010;29:633-40. [Crossref] [PubMed]
- Almufleh A, Mielniczuk LM, Zinoviev R, et al. Profound Vasoplegia During Sacubitril/Valsartan Treatment After Heart Transplantation. Can J Cardiol 2018;34:343.e5-7. [Crossref] [PubMed]
- George S, Leasure AR, Horstmanshof D. Effectiveness of Decolonization With Chlorhexidine and Mupirocin in Reducing Surgical Site Infections: A Systematic Review. Dimens Crit Care Nurs 2016;35:204-22. [Crossref] [PubMed]
- Liu Z, Norman G, Iheozor-Ejiofor Z, et al. Nasal decontamination for the prevention of surgical site infection in Staphylococcus aureus carriers. Cochrane Database Syst Rev 2017;5:CD012462. [PubMed]
- Buckberg GD, Group R. The ventricular septum: the lion of right ventricular function, and its impact on right ventricular restoration. Eur J Cardiothorac Surg 2006;29 Suppl 1:S272-8. [Crossref] [PubMed]
- Puwanant S, Hamilton KK, Klodell CT, et al. Tricuspid annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant 2008;27:1102-7. [Crossref] [PubMed]
- Potapov EV, Stepanenko A, Dandel M, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant 2008;27:1275-81. [Crossref] [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [Crossref] [PubMed]
- Morine KJ, Kiernan MS, Pham DT, et al. Pulmonary Artery Pulsatility Index Is Associated With Right Ventricular Failure After Left Ventricular Assist Device Surgery. J Card Fail 2016;22:110-6. [Crossref] [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [Crossref] [PubMed]
- Bellavia D, Iacovoni A, Scardulla C, et al. Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta-analysis of observational studies. Eur J Heart Fail 2017;19:926-46. [Crossref] [PubMed]
- Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123-30. [Crossref] [PubMed]
- Bhagra SK, Pettit S, Parameshwar J. Cardiac transplantation: indications, eligibility and current outcomes. Heart 2019;105:252-60. [Crossref] [PubMed]
- Scornik JC, Meier-Kriesche HU. Blood transfusions in organ transplant patients: mechanisms of sensitization and implications for prevention. Am J Transplant 2011;11:1785-91. [Crossref] [PubMed]
- SaBTO. Advisory Commitee on the Safety of Blood, Tissues and Organs: Cytomegalovirus tested blood components position statement. 2012. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/215125/dh_133086.pdf. Accessed 22/01/2020 2020.
- Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant 2014;33:327-40. [Crossref] [PubMed]