
Adverse events associated with prophylactic corticosteroid use before extubation: a cohort study
Introduction
Postextubation airway obstruction is an important cause of extubation failure. Reintubation is necessary for 10–100% of patients with postextubation airway obstruction or laryngeal edema (1). However, reintubation is associated with increased morbidity, mortality, and costs as well as prolonged mechanical ventilation and intensive care unit (ICU) stay (2-9). To prevent postextubation airway complications, systemic corticosteroids are used in clinical practice because they reduce laryngeal edema due to direct mucosal injury by tracheal intubation.
A clinical practice guideline published by the American Thoracic Society and American College of Chest Physicians in 2017 recommends the use of prophylactic corticosteroids in patients who failed a cuff leak test (10,11). This guideline did not fully discuss the adverse events associated with prophylactic corticosteroid use in light of postextubation airway complications. Our previous systematic review also suggested that using them before extubation reduced the incidence of postextubation airway complications and reintubation, with few adverse events (12). However, five of the 11 included studies did not address the adverse events related to prophylactic corticosteroid use, potentially representing reporting bias and precluding an adequate assessment of adverse events. Although clinicians encounter adverse events possibly associated with prophylactic corticosteroids, such as hyperglycemia, in clinical practice, the adverse events associated with prophylactic corticosteroid use are not well investigated in general.
This study aimed to describe the incidence of adverse events following the administration of prophylactic corticosteroids before extubation in mechanically ventilated adult patients. We particularly focused on the incidence of a clinically significant increase in blood glucose (BG) levels in these patients. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1790).
Methods
Study design and setting
This retrospective cohort study was conducted in the ICUs of four community-based, tertiary-care teaching hospitals, located in different areas in Japan. All participating ICUs were managed by board-certified critical care physicians. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and current ethical guidelines. The study protocol was approved by the ethics committee of each participating hospital. Individual informed consent was waived because of the retrospective nature of the study.
Participants
We included all patients who received prophylactic corticosteroids from January 1, 2014, to December 31, 2018. All participating ICUs used the same regimen of prophylactic corticosteroids: 20 mg of methylprednisolone was initiated 12 hours before the planned extubation, and the same dose was given every 4 hours with the last injection given immediately before the extubation (total dose: 80 mg), as proposed by François et al. (13) Whether to perform the cuff leak test or administer prophylactic corticosteroids was at the discretion of the treating critical care physician. We excluded patients who met any of the following criteria: (I) received systemic corticosteroids within 3 days before starting prophylactic corticosteroids according to the original indication by François et al. (13), (II) received systemic corticosteroids during the follow-up period after extubation, or (III) received prophylactic corticosteroids for the second time during an identical hospital stay.
Variables
We collected the following data from the medical charts: (I) patient characteristics [age, sex, height, weight, body mass index, presence of diabetes mellitus (DM), reason for admission, and Acute Physiology and Chronic Health Evaluation II score (14)], (II) treatment during the ICU stay (diameter of tracheal tubes, use of acid suppressants, use of vasopressors, use of systemic corticosteroids before starting prophylactic corticosteroids, use of diuretics during the initiation of prophylactic corticosteroids, use of insulin and enteral antihyperglycemic agents within 24 hours before initiating prophylactic corticosteroids, duration of mechanical ventilation, and cuff leak test results), and (III) patient outcomes (postextubation stridor, reintubation, and outcomes of interest).
We defined DM as either of the following: (I) hemoglobin A1c levels of ≥6.5%, according to the National Glycohemoglobin Standardization Program (15), or (II) an established diagnosis of DM and use of antihyperglycemic drugs including insulin. As all participating ICUs were from acute-care hospitals, it was anticipated that some patients could have been transferred to other hospitals immediately after discharge from the ICUs. Thus, we collected data on the highest BG levels within 3 days before and within the first 24 hours and the next 24–72 hours after the initiation of prophylactic corticosteroids. Similarly, we collected data on the use of steroids within 3 days of the initiation of prophylactic corticosteroids. We defined hyperglycemia as blood glucose levels of ≥180 mg/dL.
Outcomes
The primary outcome was a clinically significant increase in BG levels, defined as an increase of ≥100 mg/dL within 24 hours (0–24 hours) or 72 hours (0–72 hours) after prophylactic corticosteroids were initiated. The changes in BG levels were calculated by subtracting the BG level immediately before the initiation of prophylactic corticosteroids from the highest blood glucose level within 1 or 3 days after the drugs were initiated. We accepted all BG level measurements irrespective of whether insulin and enteral nutrition were concurrently administered during the measurement. Whether to administer insulin or antihyperglycemic drugs to patients in the peri-extubation period was at the discretion of the treating critical care physician. To be clear, we used the data of patients who had recorded BG levels within 3 days before and within 24 and/or 72 hours after the initiation of prophylactic corticosteroids. We additionally followed the BG levels of patients who experienced a clinically significant increase in BG levels as long as 7 days after initiating prophylactic corticosteroids. We captured the first timing when the BG level in such patients fell below the BG level recorded immediately before initiating prophylactic corticosteroids.
The secondary outcomes included the number of patients who newly necessitated insulin for increased BG levels after initiating prophylactic corticosteroids, the increased amount of insulin required after initiation of prophylactic corticosteroids for whom insulin had already been administrated, the incidence of new-onset infections, upper gastrointestinal (GI) bleeding, new-onset delirium, an exacerbation of existing delirium, and thrombotic events at 3 days after the initiation of prophylactic corticosteroids. We defined new-onset infections as new fever or an increase in inflammatory indices in patients with no prior infections, or superinfection in those with known infections, that was resolved with antibiotics and met one of the following criteria: (I) had confirmed microbiological evidence; or (II) had a source of infection that was suspected radiologically or on a clinical ground. We defined upper GI bleeding as one that was confirmed by upper GI endoscopy or required treatment with acid suppressants and/or transfusions. We defined new-onset delirium as a delirious status that was diagnosed using the Confusion Assessment Method for the Intensive Care Unit (16) or the Intensive Care Delirium Screening Checklist (17) or was diagnosed on a clinical ground and required treatment with antipsychotics. An exacerbation of existing delirium was defined as the use of additional antipsychotics in patients with delirium during the initiation of prophylactic corticosteroids. Moreover, we defined thrombotic events as clinically significant adverse events such as deep vein thrombosis, pulmonary embolism, or thrombosis in other organs that required treatment, because we did not routinely screen for these conditions in our practice.
Statistical analysis
We used descriptive statistics for the primary and secondary outcomes. We presented continuous variables in medians with interquartile ranges (IQRs). Subsequently, we conducted a multivariable logistic regression analysis to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the increased BG levels of ≥100 mg/dL. Fully adjusted models included variables such as age, a known diagnosis of underlying DM, concurrent use of diuretics [loop diuretics (18), thiazides (19), and glycerol (20)] with prophylactic corticosteroids, and highest blood glucose levels within 3 days prior to the initiation of prophylactic corticosteroids. Since the proportion of missing data was small, we conducted this multivariable logistic regression analysis with patients that had consistent BG level data within 3 days before and within 24 and/or 72 hours after the initiation of prophylactic corticosteroids.
We selected the above variables based on the following hypotheses. First, older ages may be associated with corticosteroid-induced hyperglycemia. A previous study suggested that age ≥60 years was associated with the incidence of DM due to corticosteroid use for a short period (21). We thus set 60 years as the cutoff of age in our study. Second, it was anticipated that the BG status in patients with DM could be exacerbated by using systemic corticosteroids, resulting in hyperglycemia. Third, glucose intolerance or insulin resistance may develop with the use of diuretics (18-20). Fourth, blood glucose levels could increase due to temporary insulin resistance and concomitant relative insulin deficiency, irrespective of the presence of underlying DM in the critically ill (22). Patients who had experienced higher BG levels in response to disease and stress are more likely to have glucose intolerance and experience higher BG levels with systemic corticosteroid use. To estimate blood glucose levels within 3 days of the initiation of prophylactic corticosteroids, we set subgroups based on the following blood glucose levels: <180, 180–359, and ≥360 mg/dL.
A two-tailed P value of <0.05 was considered statistically significant. All analyses were performed using Stata (version 15.1; StataCorp, College Station, TX, USA).
Results
Characteristics of participants
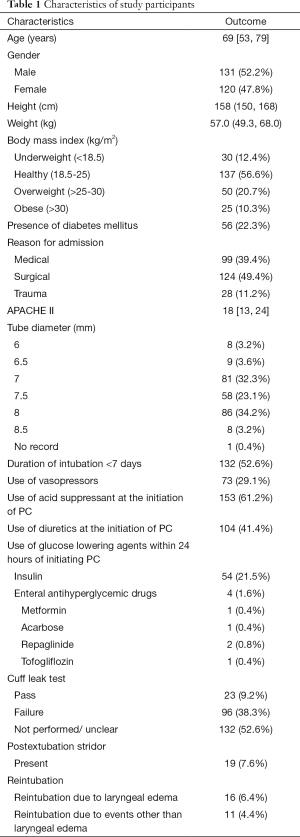
A total of 271 patients received prophylactic corticosteroids before extubation. We excluded 16 patients who received systemic corticosteroids within 3 days before starting prophylactic corticosteroids, 2 patients who received systemic corticosteroids after extubation, and 2 patients who received prophylactic corticosteroids during an identical hospital stay, thereby leaving a total of 251 patients for analysis (Figure 1). The median age was 69 years, and 131 (52.2%) patients were men (Table 1). Among the patients, 56 (22.3%) had underlying DM. The median APACHE II score was 18. Moreover, 153 (61.2%) and 104 (41.4%) patients were on acid suppressants and diuretics, respectively, during the initiation of prophylactic corticosteroids. Insulin and enteral antihyperglycemic drugs were administrated to 54 and 4 patients, respectively, within 24 hours of initiating prophylactic corticosteroids. A total of 132 patients (52.6%) received mechanical ventilation within <7 days, and out of the 119 (47.5%) who underwent the cuff leak test, 23 passed and 96 failed the test.
Full table
Primary outcomes
Data on BG levels within 3 days before and within 24 and 72 hours after the initiation of prophylactic corticosteroids were available in 248, 249, and 242 patients, respectively. The missing BG level data were due to data missing for unknown reasons. As a result, we used the data from 247 patients and 241, respectively, for the period within 24 and 72 hours after the initiation of prophylactic corticosteroids.
A total of 100 patients (40.5%) experienced hyperglycemia within 3 days before the initiation of prophylactic corticosteroids, with a median blood glucose level of 167 mg/dL. The median highest BG levels within 24 and 72 hours were 202 and 213 mg/dL, respectively (Table 2). Among the 247 patients, 230 (93.1%) experienced an increase in BG levels of any level within 24 hours after prophylactic corticosteroids were initiated, while 168 (69.7%) among 241 patients had a decline in BG levels within 24–72 hours (Table 3).

Full table

Full table
Changes in BG levels within 24 hours of initiating prophylactic corticosteroids
Out of the 247 patients, 57 (23.1%) had a clinically significant increase in BG levels (≥100 mg/dL) (Table 3). A clinically significant increase in BG levels was significantly associated with the presence of underlying DM (OR 2.65; 95% CI, 1.28–5.49) (Table 4). However, age or the use of diuretics during the initiation of prophylactic corticosteroids was not associated with a clinically significant increase in BG levels. The subgroups with blood glucose levels of 180–359 and ≥360 mg/dL had higher ORs [1.39 (95% CI, 0.70–2.77) and 3.32 (95% CI, 0.46–23.84), respectively] than the reference subgroup, although the differences were not significant.

Full table
Changes in BG levels within 72 hours of initiating prophylactic corticosteroids
Meanwhile, 73 (30.3%) out of the 241 patients showed a clinically significant increase in BG levels (Table 3). Age ≥60 years (OR 2.03; 95% CI, 1.02–4.04) and the presence of underlying DM (OR 2.47; 95% CI, 1.23–4.96) were significantly associated with a clinically significant increase in BG levels (Table 4). The subgroups with blood glucose levels of 180–359 and ≥360 mg/dL had higher ORs [1.53 (95% CI, 0.81–2.87) and 6.44 (95% CI, 0.63–65.35), respectively] than the reference subgroup, although the differences were not significant.
We additionally followed up 73 patients who experienced a clinically significant increase in BG levels (≥100 mg/dL) for the median of 3 days (IQR, 2 to 4). Within the median of 3 days (IQR, 2 to 6), the BG levels in 58 patients (79.5%) fell below the BG levels immediately before initiating prophylactic corticosteroids for the first time.
Secondary outcomes
Insulin was newly initiated in 26 patients, with the median of 5 units daily (IQR, 4 to 10; range, 2 to 22) at maximum during the 72 hours after initiating prophylactic corticosteroids. The daily dose of insulin in 54 patients who had received insulin before initiating prophylactic corticosteroids increased by 5 units (IQR, 0 to 16; range, −33 to 42).
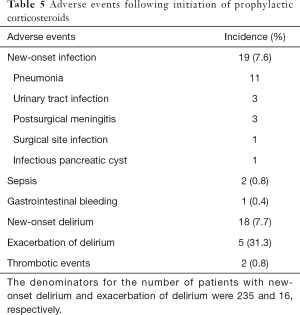
Nineteen patients (7.6%) developed new-onset infections, two of which were diagnosed as having sepsis based on the Sepsis-3 definition (23) (Table 5). The infections included pneumonia (n=11), urinary tract infection (n=3), postsurgical meningitis (n=3), an infectious pancreatic cyst (n=1), and surgical site infection (n=1). One patient (0.4%), who was not on acid suppressants, developed GI bleeding 2 days after receiving prophylactic corticosteroids. New delirious events were observed in 18 (7.7%) out of 235 patients without delirium, while the use of additional antipsychotics was observed in 5 (31.3%) out of 16 patients who had delirium after the initiation of prophylactic corticosteroids. Two patients had thrombotic events; one developed mesenteric ischemia 2 days after the initiation of prophylactic corticosteroids, and the other developed deep vein thrombosis 3 days after.
Full table
Discussion
This study showed that an increased BG level was a common adverse event of prophylactic corticosteroids. The only risk factor for such event was the presence of underlying DM, whereas patient age and the highest BG level within 3 days of initiating prophylactic corticosteroids may potentially be risk factors. New-onset infections and delirium were also common, while GI bleeding and thrombotic events were rare.
This study investigated the BG levels of patients who received prophylactic corticosteroids according to the regimen proposed by François et al. (13). François et al. originally focused on “serious” adverse events and did not report on hyperglycemia. Although hyperglycemia is generally not a serious adverse event, 70.1% of our cohort experienced BG levels of ≥180 mg/dL after the initiation of prophylactic corticosteroids, which usually indicates the blood glucose-lowering therapy in the critical care setting. Further, we found that 30.3% of the cohort experienced increased BG levels of ≥100 mg/dL within 3 days following the administration of prophylactic corticosteroids. Our study thus confirmed that hyperglycemia is a common and important adverse event of prophylactic corticosteroids.
Most recent randomized controlled trials on the use of systemic corticosteroids in the critically ill did not define hyperglycemia clearly or address hyperglycemia as an adverse event (see the online Table S1). Some of these studies also included patients with DM or hyperglycemia at baseline. Thus, in these studies, the incidence of hyperglycemia was anticipated in response to systemic corticosteroids. We assumed a priori that our patients had DM or hyperglycemia before receiving prophylactic corticosteroids. Therefore, we employed 100 mg/dL as the threshold for a clinically significant increase in BG levels rather than setting absolute criteria for hyperglycemia.
Full table
We particularly hypothesized that the blood glucose status could exacerbate in response to prophylactic corticosteroids in patients with DM and/or hyperglycemia before the initiation of prophylactic corticosteroids. Our study confirmed that the presence of underlying DM was a risk factor for a clinically significant increase in blood glucose levels. The term stress hyperglycemia has been used to define glucose intolerance in critically ill patients possibly caused by temporary insulin resistance and concomitant relative insulin deficiency in response to disease and stress (24,25). We examined the increase in BG levels based on the assumption that because patients who are ready for extubation are generally recovering from a critical state, their BG levels may be normalizing (decreasing) from stress hyperglycemia, if present. However, our study also showed that 95.9% of the cohort had increased BG levels after receiving prophylactic corticosteroids. This potentially implies that prophylactic corticosteroids might have exacerbated the condition of glucose intolerance or insulin resistance in stress hyperglycemia. Given that stress hyperglycemia or hyperglycemia in the ICU may be associated with an increased risk of incident diabetes and mortality (26), it is crucial to prevent an unnecessary increase in BG levels in the ICU. While our analysis suggested that the clinically significant increase in BG levels was not significantly associated with hyperglycemia before the initiation of prophylactic corticosteroids, the point estimate of the OR for each subgroup indicates the potential risk with wider CIs probably due to the lack of power in our study. Thus, clinicians need to be cautious about the presence of hyperglycemia following prophylactic corticosteroid use.
Further, our analysis suggested that older age (≥60 years) was associated with a clinically significant increase in BG levels within 72 hours after the initiation of prophylactic corticosteroids and was non-significantly associated with a clinically significant increase in BG levels within 24 hours after the initiation of prophylactic corticosteroids. This finding was similar to a previous study that examined the incidence of DM due to corticosteroid use for a short period (21).
Our study found that 93.1% of patients receiving prophylactic corticosteroids experienced increased BG levels within the first 24 hours of receiving the drugs, whereas 69.7% experienced a decline in BG levels during the next 48 hours. We further found that, while the BG levels in 79.5% of patients who experienced a clinically significant BG level increase could drop to the baseline BG levels, the duration could be 3 days or longer. These findings indicate that, while the majority showed a decline in BG levels after prophylactic corticosteroid use, a rise in BG levels for 3 days or longer would need careful attention. Hence, close monitoring of blood glucose levels is encouraged for at least 3 days after initiating prophylactic corticosteroids.
Systemic corticosteroids can also serve as immunosuppressants. Thus, the use of high doses of systemic corticosteroids to prevent postextubation airway complications may increase the risk of new-onset infections. However, systematic reviews of studies about the use of systemic corticosteroids for other indications showed conflicting results (27-31). In this study, approximately 8% of the cohort developed new-onset infections, which was higher than the incidence of new-onset infection reported by François et al. This emphasizes the need to monitor infectious events following prophylactic corticosteroid use.
Upper GI bleeding has been traditionally considered as another adverse event of systemic corticosteroids. Recent meta-analyses, however, suggested that systemic corticosteroids may only slightly increase the incidence of clinically significant GI bleeding in critically ill patients, with a prevalence of only 2.3–3.5% (30,32). This finding is consistent with that in our study, in which only one patient (0.4%) had upper GI bleeding, suggesting that upper GI bleeding is a rare adverse event of prophylactic corticosteroids.
In this study, new-onset delirium occurred in approximately 8% of patients who received prophylactic corticosteroids. However, there is limited evidence regarding the relationship between the development of delirium and the use of systemic corticosteroids. Previous systematic reviews suggested that neuropsychiatric events resulting from the use of systemic corticosteroids for other indications are rare, with a prevalence of 0.1–5.9% (29,30,33). The relatively high incidence of delirium in our cohort may be attributed to the fact that the patients were heterogeneous in terms of the risk of delirium.
The clinical practice guideline published in 2017 recommended giving systemic corticosteroids to mechanically ventilated patients who failed a cuff leak test (10,11). Our previous systematic review also suggested that prophylactic corticosteroids were only effective in patients who failed a cuff leak test (12). Thus, the cuff leak test is also necessary for avoiding adverse events resulting from prophylactic corticosteroid use. The 2017 guideline also recommended administering systemic corticosteroids at least 4 hours before extubation. Compared to the study by François et al., who initiated methylprednisolone (at a cumulative dose of 80 mg) 12 hours before extubation (13), Cheng et al. showed that a one-time administration of 40 mg methylprednisolone 4 hours before extubation successfully prevented postextubation airway events (34). Cheng et al. did not address adverse events due to methylprednisolone use, but it is anticipated that a smaller dose of systemic corticosteroids may be associated with the decreased incidence of adverse events including hyperglycemia. In our study, a clinically significant increase in BG levels after initiating prophylactic corticosteroids was significantly associated with the presence of underlying DM and was potentially associated with hyperglycemia before the administration of prophylactic corticosteroids. Therefore, to reduce an unnecessary increase in BG levels, lower doses of prophylactic corticosteroids need to be considered in patients with DM and hyperglycemia. Studies to compare these two regimens in terms of adverse events are warranted to further support the recommendation of the clinical practice guideline.
Our study has some limitations. First, because blood glucose levels were not measured continuously or at predetermined regular intervals, we might have used underestimated values in the analysis. Nevertheless, the impact of such limitation could have been minimized by the fact that blood glucose levels were examined every 6 hours in approximately 90% of our cohort, which was the routine practice of the participating ICUs. Second, we did not consider the severity of underlying DM, because not all patients were assessed for hemoglobin A1c levels on admission due to limited insurance coverage. Patients with higher hemoglobin A1c levels might have had higher blood glucose levels in response to systemic corticosteroid use. Third, our follow-up period may be short, although our follow-up period of 3 days was longer than that in the study by François et al., who followed up their patients for only 24 hours after extubation. Thus, we might have captured a greater incidence of adverse events associated with prophylactic corticosteroid use. We further found that the clinically significant increase in BG levels could persist for 3 days. However, the long-term adverse effects of prophylactic corticosteroids, especially transient hyperglycemia, are currently unknown and require further study. Fourth, this study did not include a no-treatment arm, making it impossible to estimate a causal association between corticosteroids and adverse events other than hyperglycemia. To our knowledge, however, this study is the first to investigate the high incidence of hyperglycemia following prophylactic corticosteroid use in the real-world setting and to suggest that prophylactic corticosteroids may have adverse events.
Conclusions
This study suggested that the use of prophylactic corticosteroids before extubation was associated with adverse events. A clinically significant increase in BG levels was the most common adverse event, with a prevalence of 30%. Lower doses of prophylactic corticosteroids may need to be considered in patients with diabetes mellitus or potential hyperglycemia to avoid adverse events. Further study to compare the different regimens of prophylactic corticosteroids is also required.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1790
Data sharing statement: Available at http://dx.doi.org/10.21037/atm-20-1790
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1790). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and current ethical guidelines. The study protocol was approved by the ethics committee of each participating hospital. Individual informed consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pluijms WA, van Mook WN, Wittekamp BH, et al. Postextubation laryngeal edema and stridor resulting in respiratory failure in critically ill adult patients: updated review. Crit Care 2015;19:295. [Crossref] [PubMed]
- Thille AW, Harrois A, Schortgen F, et al. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 2011;39:2612-8. [Crossref] [PubMed]
- Jaber S, Quintard H, Cinotti R, et al. Risk factors and outcomes for airway failure versus non-airway failure in the intensive care unit: a multicenter observational study of 1514 extubation procedures. Crit Care 2018;22:236. [Crossref] [PubMed]
- Kapnadak SG, Herndon SE, Burns SM, et al. Clinical outcomes associated with high, intermediate, and low rates of failed extubation in an intensive care unit. J Crit Care 2015;30:449-54. [Crossref] [PubMed]
- Seymour CW, Martinez A, Christie JD, et al. The outcome of extubation failure in a community hospital intensive care unit: a cohort study. Crit Care 2004;8:R322-7. [Crossref] [PubMed]
- Gowardman JR, Huntington D, Whiting J. The effect of extubation failure on outcome in a multidisciplinary Australian intensive care unit. Crit Care Resusc 2006;8:328-33. [PubMed]
- Frutos-Vivar F, Esteban A, Apezteguia C, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care 2011;26:502-9. [Crossref] [PubMed]
- Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997;112:186-92. [Crossref] [PubMed]
- Torres A, Gatell JM, Aznar E, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med 1995;152:137-41. [Crossref] [PubMed]
- Girard TD, Alhazzani W, Kress JP, et al. An Official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Rehabilitation Protocols, Ventilator Liberation Protocols, and Cuff Leak Tests. Am J Respir Crit Care Med 2017;195:120-33. [Crossref] [PubMed]
- Schmidt GA, Girard TD, Kress JP, et al. Liberation From Mechanical Ventilation in Critically Ill Adults: Executive Summary of an Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline. Chest 2017;151:160-5. [Crossref] [PubMed]
- Kuriyama A, Umakoshi N, Sun R. Prophylactic Corticosteroids for Prevention of Postextubation Stridor and Reintubation in Adults: A Systematic Review and Meta-analysis. Chest 2017;151:1002-10. [Crossref] [PubMed]
- François B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet 2007;369:1083-9. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62-9. [Crossref] [PubMed]
- Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001;29:1370-9. [Crossref] [PubMed]
- Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med 2001;27:859-64. [Crossref] [PubMed]
- Cooperman LB, Rubin IL. Toxicity of ethacrynic acid and furosemide. Am Heart J 1973;85:831-4. [Crossref] [PubMed]
- Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 2011;31:542-52. [Crossref] [PubMed]
- Frank MS, Nahata MC, Hilty MD. Glycerol: a review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy 1981;1:147-60. [Crossref] [PubMed]
- Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer 2014;22:1385-90. [Crossref] [PubMed]
- Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care 2013;17:305. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin 2001;17:107-24. [Crossref] [PubMed]
- Mifsud S, Schembri EL, Gruppetta M. Stress-induced hyperglycaemia. Br J Hosp Med (Lond) 2018;79:634-9. [Crossref] [PubMed]
- Ali Abdelhamid Y, Kar P, Finnis ME, et al. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: a systematic review and meta-analysis. Crit Care 2016;20:301. [Crossref] [PubMed]
- Fang F, Zhang Y, Tang J, et al. Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-analysis. JAMA Intern Med 2019;179:213-23. [Crossref] [PubMed]
- Rygard SL, Butler E, Granholm A, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2018;44:1003-16. [Crossref] [PubMed]
- Stern A, Skalsky K, Avni T, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev 2017;12:CD007720. [PubMed]
- Rochwerg B, Oczkowski SJ, Siemieniuk RAC, et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med 2018;46:1411-20. [Crossref] [PubMed]
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev 2019;12:CD002243. [PubMed]
- Butler E, Moller MH, Cook O, et al. The effect of systemic corticosteroids on the incidence of gastrointestinal bleeding in critically ill adults: a systematic review with meta-analysis. Intensive Care Med 2019;45:1540-9. [Crossref] [PubMed]
- Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:519-28. [Crossref] [PubMed]
- Cheng KC, Chen CM, Tan CK, et al. Methylprednisolone reduces the rates of postextubation stridor and reintubation associated with attenuated cytokine responses in critically ill patients. Minerva Anestesiol 2011;77:503-9. [PubMed]



