
Hypoproteinemia being a manifestation of immunotherapy-related liver dysfunction
Introduction
Immunotherapy has revolutionized the treatment of cancers such as melanoma, renal cancer, and non-small cell lung cancer (NSCLC) (1,2). NSCLC is a serious disease with high mortality. Recently, immunotherapeutic agents targeting the immune checkpoint pathways to enhance the anti-tumor immune reaction have shown great promise in cancer treatment and have been incorporated into the standard treatment (3). Due to checkpoint pathway-mediated immune suppression, the immune system becomes tolerant to tumor formation, and so blocking the checkpoints can activate the T cells and enhance the anti-tumor reaction (4). However, excessive immune response may cause the immune cells to attack normal tissue, leading to the occurrence of immune-related adverse events (irAEs) (1,5-11). Programmed death-1 (PD-1) is one of important immune checkpoint receptors. PD-1 is expressed in T cells, while programmed death-ligand 1 (PD-L1) is overexpressed in specific types of tumor cells (12), and the interaction of PD-1 and PD-L1 decreases T cell immune response and maintains the toleration to the tumor cells (13). Researches have demonstrated that PD-1/PD-L1 inhibitors could prolong the survival of patients with NSCLC by enhancing the immune reaction to tumor cells to take effect in the NSCLC treatment (9). A meta-analysis confirmed that patients with NSCLC receiving anti-PD-1/PD-L1 treatments had higher OS rate both in second-line therapy (HR =0.689, 95% CI: 0.635–0.747, P<0.001) and first-line therapy (HR =0.600, 95% CI: 0.407–0.884, P=0.010) than patients receiving chemotherapy (13). With the widespread use of PD-1/PD-L1 inhibitors, it is necessary to pay attention to side effects, such as pneumonitis, liver dysfunction, rash, colitis, nephritis, and endocrinopathies (14-21). Liver dysfunction is one of the most common irAEs. Most of the studies reported that hepatitis resulting from PD-1 inhibitor was manifested as elevated liver enzymes and bilirubin (22,23). Here, we report a case of immunotherapy-related liver dysfunction with hypoproteinemia. The case indicates that hypoproteinemia was manifestation of immunotherapy-related liver dysfunction. Our case also suggested that the possibility of immune-related liver dysfunction should be considered when liver damage occurs during treatment.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4980).
Case presentation
A NSCLC patient was admitted to Shanghai pulmonary hospital (Figure 1). The patient was treated with anti-PD-1 monoclonal antibody combined with chemotherapy and subsequently suffered severe hypoproteinemia and edema. And then, he progressed to grade 3 hepatic dysfunction. After receiving magnesium isoglycyrrhizinate injection to protect the liver, bile capsule to decrease bilirubin, and methylprednisolone to inhibit inflammation, the levels of liver enzyme declined, and the patient felt better. The patient had no history of liver disease, alcohol consumption, and viral hepatitis. No other hepatotoxic medications were taken during the treatment.
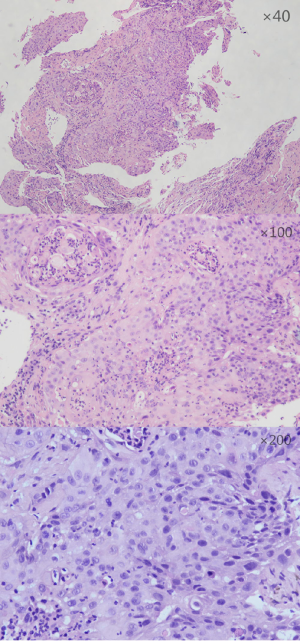
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Discussion
Immunotherapy has become a promising approach for cancer patients, with immune checkpoint inhibitors demonstrating remarkable achievements in clinical trials. Among them, PD-1/PD-L1 inhibitor can strengthen the function of effector T cells and increase antitumor reaction. At the same time, however, these inhibitors precipitate T cells to infiltrate normal tissue, resulting in irAEs, in a fashion similar to autoimmune disorder (24). The specific pathogenic mechanisms of irAEs still need to be explored. IrAEs occur in up to 70% of cancer patients with the treatment of PD-1/PD-L1 immune checkpoint inhibitors (24). A meta-analysis assessed that grade 3 and above irAEs occurred in 7.1% of cancer patients treated with PD-1 inhibitors and 6.3% treated with PD-L1 inhibitors. As for the incidence of death related to irAEs, it was reported that the death rate in patients treated with PD-1 inhibitor was higher than that in patients treated with PD-L1 inhibitor (25). When treated with checkpoint inhibitors, nearly 8–21% of patients experienced a lower degree of hepatotoxicity while grade 3–4 hepatitis was rare (9). Studies have shown that the median time of hepatic dysfunction appearing from starting PD-1 inhibitor is about 41 days (range, 21–120 days) (26).
Liver enzyme, bilirubin and serum protein were tested to observe liver function. Different from previous studies reporting that hepatitis caused by PD-1 inhibitor manifest as elevated liver enzymes and bilirubin (22,23), in this case, hypoproteinemia was the manifestation of immunotherapy-related liver dysfunction.
Albumin is synthesized and secreted by hepatocytes and is then released into extracellular space. Albumin enters into systemic circulation from interstitial space by way of the lymphatic system, with 40% of albumin being located in plasma and 60% being located in the extravascular space (27,28). PD-1 is expressed on intrahepatic T cells while PD-L1 is expressed on hepatocytes, Kupffer cells, hepatic stellate cells, and liver sinusoidal endothelial cells. The interactions between PD-1 and PD-L1 induce liver immune tolerance (29). Thus, blocking this pathway makes the autoimmune system attack the liver, which reduces the production of albumin, leading to the liver enzymes and bilirubin inside the hepatocytes being released. Patients with a serum albumin level of less than 28 g/L have been found to have a significantly increased risk of early death (30).
As immunotherapy has become a standard treatment in NSCLC, it is very important to pay attention to its adverse effects. This is the first report concerning PD-1 inhibitor–related liver dysfunction mainly manifesting as hypoproteinemia. The poor prognosis of the patient suggests that irAE of liver dysfunction, especially with hypoproteinemia, should not be ignored during treatment, and that early liver protection and glucocorticoids suppressing inflammation are necessary. Further exploration of PD-1 inhibitor–related liver dysfunction is needed to ensure oncologists perform immunotherapy more safely.
Acknowledgments
Funding: This study was supported in part by a grant of young talents in Shanghai, National Natural Science Foundation of China (81802255), Young Talents in Shanghai (2019QNBJ), ‘Dream Tutor’ Outstanding Young Talents Program (fkyq1901), Clinical Research Project of Shanghai Pulmonary Hospital (fk18005), Key Discipline in 2019 (oncology), Project of Shanghai Municipal Science and Technology Commission (Project of Municipal Science and Technology Commission), and Scientific research project of Shanghai Pulmonary Hospital (fkcx1903).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4980
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4980). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Owen D, Chaft JE. Immunotherapy in surgically resectable non-small cell lung cancer. J Thorac Dis 2018;10:S404-11. [Crossref] [PubMed]
- Salati M, Baldessari C, Cerbelli B, et al. Nivolumab in pretreated non-small cell lung cancer: continuing the immunolution. Transl Lung Cancer Res 2018;7:S91-4. [Crossref] [PubMed]
- De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181-90. [Crossref] [PubMed]
- Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. [Crossref] [PubMed]
- De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res 2017;5:312-8. [Crossref] [PubMed]
- Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 2018;6:1093-9. [Crossref] [PubMed]
- Du X, Liu M, Su J, et al. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res 2018;28:433-47. [PubMed]
- Ladak K, Bass AR. Checkpoint inhibitor-associated autoimmunity. Best Pract Res Clin Rheumatol 2018;32:781-802. [Crossref] [PubMed]
- Lerrer S, Mor A. Immune checkpoint inhibitors and the shared epitope theory: from hypothesis to practice. Transl Cancer Res 2019;8:S625-7. [Crossref]
- Liu Y, Wang H, Deng J, et al. Toxicity of tumor immune checkpoint inhibitors-more attention should be paid. Transl Lung Cancer Res 2019;8:1125-33. [Crossref] [PubMed]
- Kawakami H, Tanizaki J, Tanaka K, et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs 2017;35:529-36. [Crossref] [PubMed]
- Zeng T, Qin Q, Bian Z, et al. Clinical efficacy and safety of anti-PD-1/PD-L1 treatments in non-small cell lung cancer (NSCLC). Artif Cells Nanomed Biotechnol 2019;47:4194-201. [Crossref] [PubMed]
- Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One 2016;11:e0160221. [Crossref] [PubMed]
- Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43-50. [Crossref] [PubMed]
- Cukier P, Santini FC, Scaranti M, et al. Endocrine side effects of cancer immunotherapy. Endocr Relat Cancer 2017;24:T331-47. [Crossref] [PubMed]
- Kumar V, Chaudhary N, Garg M, et al. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol 2017;8:49. [Crossref] [PubMed]
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [Crossref] [PubMed]
- Sznol M, Postow MA, Davies MJ, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70-6. [Crossref] [PubMed]
- Wang PF, Chen Y, Song SY, et al. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol 2017;8:730. [Crossref] [PubMed]
- Weber JS, Postow M, Lao CD, et al. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist 2016;21:1230-40. [Crossref] [PubMed]
- Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 2013;31:1071-7. [Crossref] [PubMed]
- Zhang HC, Luo W, Wang Y. Acute liver injury in the context of immune checkpoint inhibitor-related colitis treated with infliximab. J Immunother Cancer 2019;7:47. [Crossref] [PubMed]
- Stucci S, Palmirotta R, Passarelli A, et al. Immune-related adverse events during anticancer immunotherapy: Pathogenesis and management. Oncol Lett 2017;14:5671-80. [PubMed]
- El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 2017;119:1-12. [Crossref] [PubMed]
- Mathew Thomas V, Bindal P, Ann Alexander S, McDonald K. Nivolumab-induced hepatitis: A rare side effect of an immune check point inhibitor. J Oncol Pharm Pract 2020;26:459-61. [Crossref] [PubMed]
- Artigas A, Wernerman J, Arroyo V, et al. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care 2016;33:62-70. [Crossref] [PubMed]
- Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836-46. [Crossref] [PubMed]
- Cho H, Kang H, Lee HH, et al. Programmed Cell Death 1 (PD-1) and Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA-4) in Viral Hepatitis. Int J Mol Sci 2017;18:1517. [Crossref] [PubMed]
- Chang PE, Goh GB, Tan CK. Low serum albumin and advanced age predict early mortality in Asian patients with extreme elevations of serum aminotransferase. J Dig Dis 2016;17:193-201. [Crossref] [PubMed]


