
Quantitative CT measurement of left colonic and pelvic mesenteric adipose volume in radiation proctitis
Introduction
Preoperative radiotherapy combined with concurrent chemotherapy is the standard neoadjuvant regimen for locally advanced rectal cancer (LARC) (1). Studies have demonstrated neoadjuvant chemoradiotherapy can reduce the tumor stage, prevent local recurrence, and improve the rate of anus preservation (2,3). But these advances have not markedly decreased the risk of distant metastasis, which remains the leading cause of death for LARC. Recently, multiple trials have reported promising outcomes on the use of total neoadjuvant therapy (TNT), incorporating both chemotherapy and radiotherapy in the preoperative setting. With improved treatment compliance and reduced toxicities, TNT increases the pathologic complete response rate and facilitates patients who are eligible for organ preservation, potentially increases distant control and long-term survival for patients with LARC (4-7).
However, there are potential disadvantages after neoadjuvant chemoradiotherapy. The delay in definitive surgery could allow for local disease progression, particularly in those patients who do not respond to neoadjuvant treatment. Neoadjuvant chemoradiotherapy may impact the performance status of patients who undergo planned surgery, which potentially increase postoperative complications. Higher grade 3 to 4 radiation dermatitis, hematologic, and gastrointestinal toxic effects were observed in patients who received radiotherapy (8-10). Radiation proctitis (RP), caused by preoperative radiotherapy, can profoundly reduce the patients’ anal functions and quality of life (11-13). Randomized controlled trials and post hoc analyses have shown that preoperative radiotherapy greatly increases the incidence of anastomotic leakage, and that RP manifest in preoperative imaging is an independent risk factor for anastomotic leakage (8,14).
RP is an inflammatory bowel disease diagnosed through a combination of clinical, endoscopic, and imaging measures. The most common and bothersome complaint from RP patients is rectal bleeding. Other symptoms include anal pain, diarrhea, urgency, and incontinence (15). Endoscopic biopsy is performed for differential diagnosis and to rule out other causes of chronic proctitis (16). Surgical assessment of RP often reveals that the mesentery is edematous and thickened, but the relevance of these features has yet to be determined.
The previous study investigated the effect of radiation-induced injury on anastomosis from pathological view and found that RP had a more severe microvascular injury (17). Preoperative evaluation of RP is helpful to identify patients at high risks of anastomotic leakage. Computed tomography (CT) occupies an important role in evaluating bowel radiation injury, and typical CT manifestations include thickening of the rectal wall and edema of mesenteric adipose tissues. To the best of our knowledge, there is no unified quantitative standard to evaluate the change of mesenteric adipose in RP. The purposes of this study were thus to quantitatively measure the variation of left colonic and pelvic mesenteric adipose volume after radiotherapy, to analyze its correlation with the thickening degree of the rectal wall, and to evaluate the ability of mesenteric adipose volume changes to identify RP.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5102).
Methods
Patients
From March 2013 to June 2015, the data from patients with locally advanced rectal cancer who were enrolled in an open-label, multicenter, three-arm randomized controlled trial (FOWARC study, NCT01211210) at the Sixth Affiliated Hospital of Sun Yat-sen University for neoadjuvant chemoradiotherapy were retrieved. The detailed protocol has been published elsewhere (8).
Inclusion criteria in this study were patients who received a total dose of 46.0–50.4 Gy administered 5 times weekly in 25 to 28 daily fractions, along with concurrent fluorouracil-based chemotherapy; patients who underwent radical operation for rectal cancer 4 to 6 weeks after chemoradiotherapy; and patients with complete abdominal and pelvic enhanced CT image data before and after radiotherapy. Meanwhile, patients with incomplete CT images, including those whose original data were broken or unavailable, were excluded.
Ethical approval and patient informed consent are waived as we analyzed the CT images retrospectively, without any interventions or hazard to patients.
Diagnosis of RP
RP was diagnosed by clinical history, preoperative colonoscopy, and radiologic investigation. Endoscopic manifestations include telangiectasia, diffuse mucosal edema, ulcer, bowel stenosis, and stiffness. The main manifestations of pelvic magnetic resonance imaging (MRI) are edema and circumferential thickening of the rectal wall, accompanied by stratification effect, edema of the mesentery and surrounding pelvic soft tissues, and similar tissue changes of the distal sigmoid colon within the scope of radiotherapy (14,18).
CT protocols
CT images were obtained through the Toshiba Aquilion ONE 128-detector CT scanner. The scanning range was from the lower third of the heart to the pubic symphysis. The tube voltage was 120 KV, and the tube current was 330 mAs. The contrast agent was injected through the cubital vein with an injection flow rate of 3.0 mL/s for enhanced scanning. All slides were reconstructed into images with 3 mm layer thickness and 3 mm layer spacing.
Measurement of left colonic and pelvic mesenteric adipose volume
The reconstructed images were analyzed according to the ANYTHINK GVCM system (CREALIFE Medical Technology, Beijing, China). The left colonic mesenteric adipose volume was measured in each vertebral space from the third lumbar vertebra to the first sacral vertebra (L3 to S1). The upper boundary of the pelvic mesenteric adipose volume was at the level of the S1 vertebra, and the lower boundary was at the hiatus of the levator ani muscle (Figure 1).
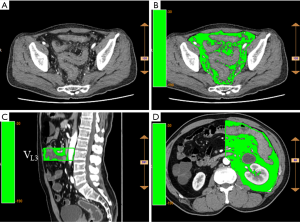
All measurements were performed by the same senior physician. To ensure blinding, the grouping information of the patients was not divulged before measurement.
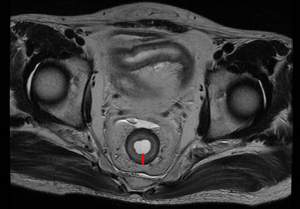
Measurement of rectal wall thickness
Pelvic MRI was used to measure the thickness of the rectal wall without tumor (Figure 2). All of the recruited patients received an initial MRI scan before treatment for tumor staging and a second MRI scan after completion of neoadjuvant chemoradiotherapy. The MRI protocols and measurement have been reported previously (19). The data on rectal wall thickness was collected from the RP database for analysis.
Statistics
Continuous data are represented as “ ”, and categorical data as proportions (percentage). The Student’s t-test was applied to evaluate the differences in continuous variables depending on the normality of the data distribution. The Chi-square test or Fisher’s exact test was utilized to evaluate differences in categorical variables, as appropriate. A two-sided Pearson’s test was adopted for correlation regression analysis. SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was employed for analysis. Differences were proven statistically significant if the P value was <0.05.
For evaluation, the relative change of mesenteric adipose volume (ΔV%) was defined as follows: (mesenteric adipose volume after chemoradiotherapy − mesenteric adipose volume before chemoradiotherapy)/mesenteric adipose volume before chemoradiotherapy × 100%. The relative change of rectal wall thickness (ΔL%) was calculated as follows: (rectal wall thickness after chemoradiotherapy −rectal wall thickness before chemoradiotherapy)/rectal wall thickness before chemoradiotherapy × 100%.
Results
Patient characteristics
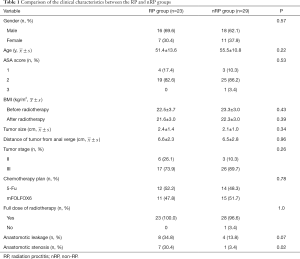
A total of 52 patients were recruited in this study, including 23 cases of RP (RP group) and 29 cases of non-RP (nRP group). The patients in the RP group tended to have more anastomotic leakage (both clinical and subclinical leaks) than those in the nRP group [34.8% (8/23) vs. 13.8% (4/29), P=0.07]. The rate of anastomotic stenosis was considerably higher in the RP group than in the nRP group [30.4% (7/23) vs. 3.4% (1/29), P=0.02]. The other clinical characteristics between the two groups were comparable (Table 1).
Full table
CT measurement
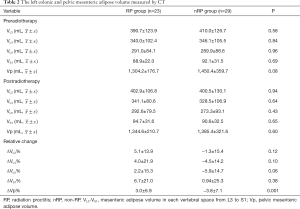
Table 2 presents the left colonic and pelvic mesenteric adipose volume measurement results. There was no significant difference in the left colonic mesenteric adipose volume before and after neoadjuvant radiotherapy in each vertebral space from L3 to S1 between the two groups, neither was the pelvic mesenteric adipose volume. Concerning the relative change of mesenteric adipose volume, the increment of the relative change of pelvic mesenteric adipose volume (ΔVp%) was greater in the RP group than in the nRP group (P=0.001).
Full table
Predictive value of ΔVp% in RP
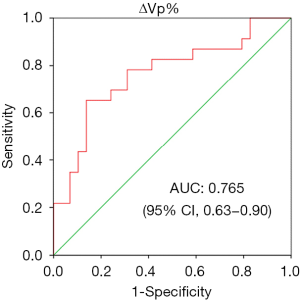
The ΔVp% was additionally introduced into the receiver operating characteristic (ROC) curve to evaluate the predictive value for RP (Figure 3). The area under the curve (AUC) was 0.765, which displayed a significant value in differentiating RP from nRP (P<0.001). With a cutoff value of 3.67%, the sensitivity and specificity for the diagnosis of RP were 65.2% and 86.2%, respectively.
Correlation between ΔVp% and ΔL%
The maximum diameter of the rectal wall thickness of the two groups, not including the location of the tumor, was measured on the T2 axial image of the pelvic MRI before and after radiotherapy (Table 3). After radiotherapy, the thickness of the rectal wall was markedly increased in the RP group. The relative change of rectal wall thickness (ΔL%) was more observable in the RP group than in the nRP group (P<0.01).

Full table
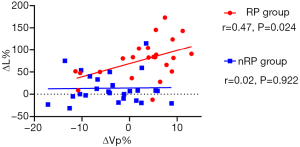
ΔVp% and ΔL% were additionally included in the correlation analysis. The correlation coefficient in the RP group of 0.47 and the two-sided Pearson’s test outcome of P=0.024 implied that ΔVp% was notably correlated with ΔL%, whereas there was no statistically observable correlation in the nRP group (r=0.02, P=0.922) (Figure 4).
Discussion
RP is a common consequence of radiation therapy for pelvic malignancies (20,21). Efforts have been made to specifically prevent, diagnose, and manage its adverse effects (22). However, no gold standard exists for RP diagnosis. In the present study, the left colonic and pelvic mesenteric adipose volume were quantitatively measured, and their relation to RP was analyzed. The ΔVp% was dramatically higher in the RP group, with a favorable sensitivity and specificity in predicting RP. Moreover, ΔVp% and ΔL% were highly correlated. For patients undergoing neoadjuvant chemoradiotherapy, ΔVp% could be regarded as a useful predictor of RP.
The mesentery is considered to be an independent organ, and thus its function and role in various diseases have become issues of intense research interest (23,24). Histological study has confirmed that the epithelial cells and connective tissue of the mesentery are continuous with the intestinal wall (25). The abnormalities of mesenteric adipose tissue morphology and function are closely bound up with the occurrence and development of various diseases (26-28). In RP, when the pelvic mesentery is exposed to radiation, it is inevitably injured. The mesentery may appear to present such imaging changes as edema, increased density, thickening of mesenteric vessels, and blurred margin after radiotherapy. As far as we know, few if any studies have investigated the relationship between mesenteric adipose volume and radiation injury. We therefore believe that the present study is the first to use CT to quantitatively measure the mesenteric adipose volume before and after radiotherapy. All measurements of this study were performed by the same senior physician to control bias. We discovered that ΔVp% had a fair ability to predict RP. While there was no prominent difference in pelvic mesenteric adipose volume before and after radiotherapy between the RP group and the nRP group, ΔVp% in the RP group was markedly higher than that in the nRP group. The decrease of ΔVp% in the nRP group may be related to the reduction in body mass index (BMI). Owing to the influence of long-course chemoradiotherapy and tumor burden, most patients experience a decline in BMI after a short period of time. Also, the intestinal symptoms may put patients under extreme psychological pressure, significantly reducing their food intake, and eventually increasing the incidence of malnutrition in radiation enteritis patients (29). Therefore, more attention should be paid to the nutrition of radiotherapy patients. Second, the left colonic mesenteric adipose volume in each vertebral space from L3 to S1 showed no difference across the RP and nRP groups. This indicates that the effect of radiotherapy for locally advanced rectal cancer on the mesentery was mainly below the S1 plane of the pelvis. As the previous study suggested, part of the sigmoid colon, which can be used for anastomosis in a typical resection, is still within the irradiation field (19,30), and so the distal rectum is inevitably exposed to radiation injury. It was found that the use of a nonirradiated bowel for at least one end of an anastomosis substantially lowered the anastomotic leakage rate (31). Hence, we suggest that the scope of proximal bowel resection should be above the S1 level in radical operation for patients with RP.
CT or MRI has been used to examine the area of adipose tissues on a single cross-section passing through the umbilicus in order to evaluate the total abdominal adipose content and the visceral adipose content (32-35). However, for rectal cancer patients receiving radiotherapy, this method has failed in measuring the ΔVp%. In this study, the reconstructed images were analyzed with the ANYTHINK GVCM system, through which we could continuously delineate the mesenteric area in any plane, and then calculate the total mesenteric adipose volume in the target area. This measurement has high reproducibility and provides a quantitative evaluation basis for comparing changes in abdominal and ΔVp%.
Manifestations in typical images of RP can include the thickening of the rectal wall and the obvious thickening of the mucosa and/or serosal layer, which present a stratified change or “target sign” (36). In the previous study, T2 cross-sectional MRI images of the pelvis were used to measure the maximum diameter of rectal wall thickness, excluding the tumor location, before and after radiotherapy (19). The data of rectal wall thickness were collected from the radiation enteritis database for analysis. Compared with the nRP group, the relative change of rectal wall thickness (ΔL%) in the RP group was greater. The correlation analysis indicated that ΔVp% was highly correlated with ΔL% in the RP group, but this was not the case for the nRP group. This confirms that ΔVp% and ΔL% possess synergistic diagnostic values for RP.
The FOWARC study included 495 locally advanced rectal cancer patients who received neoadjuvant treatment and were randomly assigned to the mFolfox6 chemotherapy group, the fluorouracil + radiotherapy group, or the mFolfox6 + radiotherapy group. Study results revealed that the incidence of anastomotic leakage was 7.9%, 19.8%, and 18.1% respectively in the three groups, suggesting that preoperative neoadjuvant radiotherapy substantially increases the incidence of postoperative anastomotic leakage (8). In our study, anastomotic leakage occurred in 8 of 23 patients (34.8%) in the RP group and 4 of 29 patients (13.8%) in the nRP group. The incidence of anastomotic stenosis in the RP group was markedly higher than that in the nRP group (30.4% vs. 3.4%, P=0.02), implying that RP was a risk factor for anastomotic leakage and stenosis.
There are also some limitations in this study. Firstly, this was a retrospective study, and the sample size was relatively small. Secondly, the mesenteric adipose volume was measured by manual sketching, which might have inherently led to random errors. The development of artificial intelligence and the application of the computer to sketch automatically will be conducive to reducing these inconsistencies.
Conclusions
The ΔVp% which correlated with the thickness of the rectal wall, demonstrated a satisfactory sensitivity and specificity in diagnosing RP. Apply CT to quantitative measurement of the ΔVp% is thus viable as a simple and noninvasive evaluation method.
Acknowledgments
The authors would like to thank Rong Zhang for software support.
Funding: This work was supported by the Natural Science Foundation of Guangdong Province (No. 2018A030310319).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5102
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5102
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5102). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval and patient informed consent are waived as we analyzed the CT images retrospectively, without any interventions or hazard to patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benson AB, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:874-901. [Crossref] [PubMed]
- Zhou C, Liu HS, Liu XH, et al. Preoperative assessment of lymph node metastasis in clinically node-negative rectal cancer patients based on a nomogram consisting of five clinical factors. Ann Transl Med 2019;7:543. [Crossref] [PubMed]
- Ferrari L, Fichera A. Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep (Oxf) 2015;3:277-88. [PubMed]
- Perez K, Safran H, Sikov W, et al. Complete Neoadjuvant Treatment for Rectal Cancer: The Brown University Oncology Group CONTRE Study. Am J Clin Oncol 2017;40:283-7. [Crossref] [PubMed]
- Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957-66. [Crossref] [PubMed]
- Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trialdagger. Ann Oncol 2015;26:1722-8. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Sabbaga J, et al. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum 2009;52:1927-34. [Crossref] [PubMed]
- Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol 2016;34:3300-7. [Crossref] [PubMed]
- Eisterer W, Piringer G. Neoadjuvant Chemotherapy with Capecitabine, Oxaliplatin and Bevacizumab Followed by Concomitant Chemoradiation and Surgical Resection in Locally Advanced Rectal Cancer with High Risk of Recurrence - A Phase II Study. Anticancer Res 2017;37:2683-91. [Crossref] [PubMed]
- Sauer R, Fietkau R, Wittekind C, et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis 2003;5:406-15. [Crossref] [PubMed]
- Battersby NJ, Juul T, Christensen P, et al. Predicting the Risk of Bowel-Related Quality-of-Life Impairment After Restorative Resection for Rectal Cancer: A Multicenter Cross-Sectional Study. Dis Colon Rectum 2016;59:270-80. [Crossref] [PubMed]
- Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol 2007;8:1007-17. [Crossref] [PubMed]
- Qin Q, Huang B, Cao W, et al. Bowel Dysfunction After Low Anterior Resection With Neoadjuvant Chemoradiotherapy or Chemotherapy Alone for Rectal Cancer: A Cross-Sectional Study from China. Dis Colon Rectum 2017;60:697-705. [Crossref] [PubMed]
- Qin Q, Ma T, Deng Y, et al. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis After Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis Colon Rectum 2016;59:934-42. [Crossref] [PubMed]
- Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19:790-9. [Crossref] [PubMed]
- Wu XR, Liu XL, Katz S, et al. Pathogenesis, diagnosis, and management of ulcerative proctitis, chronic radiation proctopathy, and diversion proctitis. Inflamm Bowel Dis 2015;21:703-15. [Crossref] [PubMed]
- Zhong QH, Wu PH, Qin QY, et al. Zhonghua Wai Ke Za Zhi 2017;55:507-14. [Pathological insights of radiotherapy-related damage to surgical margin after preoperative radiotherapy in patients with rectal cancer]. [PubMed]
- Sugimura K, Carrington BM, Quivey JM, et al. Postirradiation changes in the pelvis: assessment with MR imaging. Radiology 1990;175:805-13. [Crossref] [PubMed]
- Ma T, Zhong Q, Cao W, et al. Clinical Anastomotic Leakage After Rectal Cancer Resection Can Be Predicted by Pelvic Anatomic Features on Preoperative MRI Scans: A Secondary Analysis of a Randomized Controlled Trial. Dis Colon Rectum 2019;62:1326-35. [Crossref] [PubMed]
- Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol 2014;11:470-9. [Crossref] [PubMed]
- Hayne D, Vaizey CJ, Boulos PB. Anorectal injury following pelvic radiotherapy. Br J Surg 2001;88:1037-48. [Crossref] [PubMed]
- Paquette IM, Vogel JD, Abbas MA, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis. Dis Colon Rectum 2018;61:1135-40. [Crossref] [PubMed]
- Coffey JC, O'Leary DP. The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol 2016;1:238-47. [Crossref] [PubMed]
- Coffey JC, O'Leary D P. Defining the mesentery as an organ and what this means for understanding its roles in digestive disorders. Expert Rev Gastroenterol Hepatol 2017;11:703-5. [Crossref] [PubMed]
- Walsh LG, O'Brien IS, O'Leary DP, et al. The mesocolic hilum: an electron microscopic appraisal of anatomy. Irish Journal of Medical Science 2016;185:S97-S.
- Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut 2007;56:577-83. [Crossref] [PubMed]
- Tracy RP. Is visceral adiposity the "enemy within"? Arterioscler Thromb Vasc Biol 2001;21:881-3. [Crossref] [PubMed]
- Bonen A, Tandon NN, Glatz JF, et al. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond) 2006;30:877-83. [Crossref] [PubMed]
- Zhu W, Gong J, Li Y, et al. A retrospective study of surgical treatment of chronic radiation enteritis. J Surg Oncol 2012;105:632-6. [Crossref] [PubMed]
- Qin Q, Zhu Y, Wu P, et al. Radiation-induced injury on surgical margins: a clue to anastomotic leakage after rectal-cancer resection with neoadjuvant chemoradiotherapy? Gastroenterol Rep (Oxf) 2019;7:98-106. [Crossref] [PubMed]
- Galland RB, Spencer J. Surgical management of radiation enteritis. Surgery 1986;99:133-9. [PubMed]
- van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 1993;17:187-96. [PubMed]
- Weits T, van der Beek EJ, Wedel M, et al. Computed tomography measurement of abdominal fat deposition in relation to anthropometry. Int J Obes 1988;12:217-25. [PubMed]
- Terry JG, Hinson WH, Evans GW, et al. Evaluation of magnetic resonance imaging for quantification of intraabdominal fat in human beings by spin-echo and inversion-recovery protocols. Am J Clin Nutr 1995;62:297-301. [Crossref] [PubMed]
- Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology 1999;117:73-81. [Crossref] [PubMed]
- Algin O, Turkbey B, Ozmen E, et al. Magnetic resonance enterography findings of chronic radiation enteritis. Cancer Imaging 2011;11:189-94. [PubMed]


