
Lung parenchymal and airway changes on CT imaging following allergen challenge and bronchoalveolar lavage in atopic and asthmatic subjects
Introduction
Asthma is among the most common lung diseases in the world, with expected prevalence of 400 million people by 2025 (1). It is characterized by bronchial epithelial hyper-responsiveness to an external trigger or allergen resulting in “variable and recurring symptoms” such as wheezing, shortness of breath, and cough due to airflow obstruction (2-4). While atopic, non-asthmatics may have allergic symptoms including rhinorrhea or sinonasal congestions following exposure to an allergen, obstructive airway symptoms are typically absent, and the mechanism behind this disparity in symptoms between the two groups remains unclear.
Attempts have been made to characterize differences in disease activity among asthmatic and non-asthmatic atopic subjects using functional imaging following experimentally induced inflammation by installing an allergen in the lung segment through bronchoscopy. Positron emission tomography (PET) has been used to demonstrate differences in 18F-fluorodeoxyglucose (18F-FDG) uptake, and functional imaging has been used to assess the ventilation and perfusion in these segments (5-8). Quantitative assessment of the airways using optical coherence tomography has been used to demonstrate remodeling in asthmatic patients as a reaction to the same allergen challenge (9). These modalities, however, are currently of limited clinical utility in the management of asthma. We, therefore, sought to determine the effect of locally administered allergen on routine computed tomography (CT) of the chest.
The purpose of this study was to characterize the CT appearance of the airways and lung parenchyma in asthmatic and non-asthmatic atopic subjects following a segmental allergen challenge. As a secondary goal, we sought to characterize the imaging changes following bronchoalveolar lavage (BAL) in subjects with atopy with and without asthma. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1719).
Methods
Subject selection
This prospective observational (cohort) study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by institutional review board of Partners Healthcare (IRB2008P000408) under a protocol previously described (5). Informed consent was taken from all the patients.
We recruited a total of 20 volunteers with atopy based on clinical evaluation, documented allergic symptoms to cat and/or dust mite, and a positive skin prick test. Of these, 10 subjects were non-asthmatic and 10 subjects were asthmatic based on clinical evaluation. The asthmatic subjects had mild or moderate persistent asthma based on the National Asthma Education and Prevention Program (NAEPP) management guidelines (3). All subjects were free of any acute respiratory symptoms and had been off of any corticosteroids (including inhaled corticosteroids) for at least 1 month and short acting beta-agonists or antihistamines were held at least 24 hours prior to intervention.
BAL and segmental allergen challenge
Fiberoptic bronchoscopy was performed under conscious sedation and topical anesthesia. Three different interventions on three separate lung segments were performed: first, BAL was performed in the lingula with instillation and aspiration of 120 mL of normal saline. Second, allergen mixed with diluent (2 mL total) was instilled in the lateral segment of the right middle lobe. The allergen used was a standardized extract of either Dermatophagoides pteronyssinus, Dermatophagoides farina, or cat hair. Finally, a 2 mL aliquot of inert diluent without the allergen was instilled in the anterior segment of the right upper lobe.
Image acquisition and analysis
All subjects had a non-contrast CT of the chest approximately 10 hours following the interventions. The timing of the CT was selected to fit into our research workflow and to ensure uniformity across subjects. All CTs were performed using a 64-detector row scanner (Biograph 64; Siemens Healthcare, Erlangen, Germany). CT was performed with 120 kVp and tube current up to 160 mAs, and images reconstructed using a B31f kernel and 0.75 mm slice thickness with 0.25 mm overlap. Images were analyzed on Pulmonary Workstation 2 (VIDA Diagnostics) by two thoracic radiologists (S.D. and J.N.), with findings determined and recorded by consensus.
Images were evaluated for abnormalities of the airways, parenchyma, and thoracic lymph nodes. Bronchial wall thickening was graded as none, mild (<25% width of the bronchial lumen), moderate (25–50% width of the bronchial lumen), or severe (>50% width of the bronchial lumen). Bronchiectasis was recorded as present or absent, and small airways were considered to be involved when tree-in-bud nodules or centrilobular nodules were present. Centrilobular nodules are round opacities centered on the respiratory bronchioles, and tree-in-bud nodules are branching opacities that assume “V” and “Y” morphologies typically due to intraluminal secretions or mucus in smaller, more distal bronchioles. Parenchymal involvement was classified as either consolidation or ground-glass opacity. Consolidation was defined as “homogeneous increase in pulmonary parenchymal attenuation that obscures the margins of vessels and airway walls”, while ground-glass was defined as “hazy increased opacity of lung, with preservation of bronchial and vascular margins (10).” Interlobular septal thickening was recorded as present or absent. Hilar and peribronchial lymphadenopathy was also recorded as present or absent. The lymph nodes were measured in short axis and nodes greater than 1 cm in shot axis are considered enlarged as per standard convention.
Categorical variables were reported as absolute values with frequencies. Fisher’s exact test was used for comparison of categorical variables among groups. All tests were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
We included 20 atopic volunteer subjects (median age: 23.5 years, range: 18–48 years; female =11, male =9) who were either asthmatic (AA, n=10) or non-asthmatic (ANA, n=10). Included subjects are summarized in Table 1.
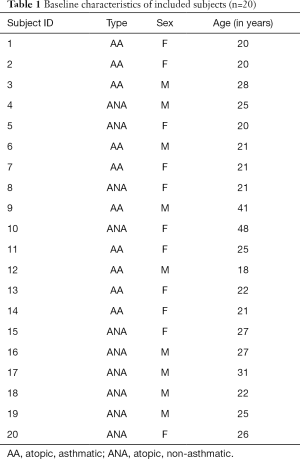
Full table
Post-intervention imaging features
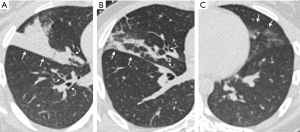
CT imaging changes following BAL and instilling of the allergen (AL) and the inert diluent (DL), respectively, are summarized in Table 2 and Figure 1. The most common finding following the allergen challenge was moderate to severe bronchial wall thickening (14/20; 70%), which was more common than following BAL (0/20, 0%; P<0.01) or following diluent instillation (0/20, 0%; P<0.01). Consolidations following allergen challenge (11/20; 55%) were more common compared to following BAL or diluent (BAL=3/20, 15%, P=0.02; DL=0/20, 0%, P<0.01). Interlobular septal thickening was seen in 7/20 (35%) subjects following allergen instilling and was not observed following either BAL or diluent (P<0.01). Representative CT images following the allergen challenge are shown in Figure 2.

Full table
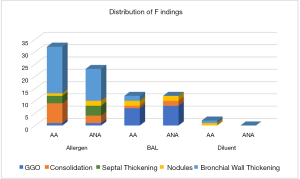
In contrast, the most common finding following BAL in all subjects is localized ground-glass opacity (15/20; 75%), which was significantly more frequent than following the instillation of allergen (2/20, 10%; P<0.01) or diluent (0/20, 0%; P<0.01). When parenchymal involvement is present (n=18), it typically involved less than 50% of the affected segment (15/18; 83.3%). Mild bronchial wall thickening (2/20; 10%) and tree-in-bud or centrilobular nodule formation (1/20; 5%) were uncommon. No subject had bronchiectasis or lymphadenopathy following BAL (Table 2).
Airway or parenchymal changes were uncommon following instillation of the diluent, which only resulted in mild bronchial wall thickening in one asthmatic subject (1/20) and nodule formation in one other asthmatic subject (1/20).
Changes in asthmatics versus non-asthmatics
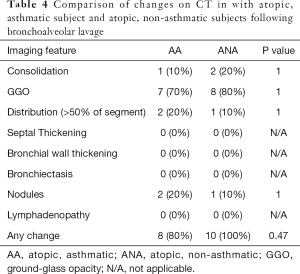
Compared to the non-asthmatic subject, asthmatic subjects had increased incidence of consolidation following the allergen challenge, although this did not reach statistical significance (AA=8/10, 80% vs. ANA=3/10, 30%; P=0.07). Significant (moderate or severe) bronchial wall thickening was seen in both asthmatics and non-asthmatics (AA=7/10, 70% vs. ANA=6/10, 60%; P=1). Severe bronchial wall thickening was more common in asthmatics but did this not reach statistical significance (AA=4/10, 40% vs. ANA=1/10, 10%; P=0.3). Three subjects (subjects 8, 10, and 16) had no airway or lung parenchymal changes following the allergen challenge, and all of these subjects were non-asthmatics. There was, otherwise, no significant difference between the two groups with regards to parenchymal or airway changes with either instillation of the allergen or following BAL. Comparisons between the two subgroups are summarized in Tables 3 and 4 and Figure 1.
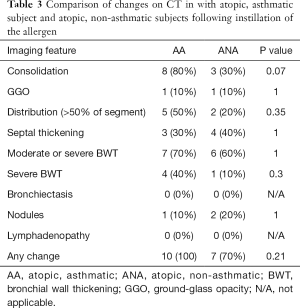
Full table
Full table
Discussion
Our study is the first to systematically analyze the CT imaging findings in the lungs and airways following a localized allergen challenge in atopic patients and also the first study to characterize the imaging changes on CT resulting from BAL in these patients. Our findings suggest that exposure of the lungs to allergens can result in significant localized bronchial wall thickening, consolidative opacities, and septal thickening in atopic patients regardless of asthma status, but that there may be increased tendency for asthmatics to have consolidations compared to non-asthmatics. Also, we found that routine BAL can result in the development of ground-glass opacities in those with atopy.
The radiologic findings of asthma are well described in both plain chest radiography and CT and most notably include hyperinflation and bronchial wall thickening with variable mucous plugging, atelectasis, and air trapping (11-17). These assessments, however, have typically been performed in the setting of chronic, severe asthma or the setting of acute exacerbation or presumed infection. The direct effect of the allergen to the lung parenchyma and airways on CT has not been described.
In our cohort, most of the subjects developed moderate to severe bronchial wall thickening following instillation of the allergen (Figure 2). This was seen in both asthmatic and non-asthmatic subjects. In those who had bronchial wall thickening, severe bronchial wall thickening was more frequently observed in asthmatic subjects compared to non-asthmatic subjects. Notably, three out of the 20 subjects did not have any airway or lung parenchymal changes following the allergen challenge. All three subjects were non-asthmatics, while all the asthmatic subjects had airway or lung parenchymal changes following the allergen challenge. These differences, however, did not reach statistical significance likely due to the small sample size.
The degree of bronchial wall thickening has previously been correlated with the duration, severity, and degree of airflow limitation in asthma (13,14). Asthma affects both central and peripheral airways, and the resultant bronchial wall thickening is thought to be secondary to both acute and chronic inflammatory changes and downstream structural remodeling (18,19). Inflammatory changes in the airway involve activation of eosinophils, CD4 T-lymphocytes, and mast cells, among others, with eosinophilic infiltration, thought to be the hallmark of the disease (18,20,21). Downstream airway changes include collagen deposition, increased smooth muscle mass, glandular hypertrophy, and vascular congestion (19,22-25). Given the relatively rapid (approximately 10 hours) development of bronchial wall thickening in our subjects, the findings are likely due to the acute inflammatory process and edema rather than actual structural remodeling.
In addition to bronchial wall thickening, more than half of our subjects had consolidations following exposure to the allergen (Figure 2) and were more frequently observed in asthmatics. The consolidations observed are likely multifactorial. Bronchial wall thickening and resultant luminal narrowing obstruction may contribute to post-obstructive consolidation. The consolidations are also likely a result of inflammatory cellular infiltration and mucus hypersecretion, which can be seen shortly following allergen exposure (26-28). Both bronchial wall thickening and consolidations are common imaging findings in pneumonia and other infections, but these findings should be interpreted with caution in patients who present with acute asthma exacerbation, particularly if there are no other clinical findings to suggest an underlying infection.
Another imaging feature seen following the allergen challenge was septal thickening (Figure 2). This feature was seen in both asthmatic and non-asthmatic subjects following allergen challenge, but was not seen following instillation of the inert diluent or BAL. We suspect that the septal thickening is related to localized inflammatory edema and increased fluid along the pulmonary interstitial lymphatics.
None of the subjects (not even the asthmatics) in our cohort developed bronchiectasis following the allergen challenge. Bronchiectasis is reported to be a common finding in severe asthma, with a study reporting 77% of patients having at least one dilated bronchus (29,30). Bronchiectasis in asthmatic patients has also been associated with more frequent hospitalizations (31). The absence of bronchiectasis in any of our subjects is likely multifactorial. We only included patients with mild or moderate asthma in this study. Additionally, we only exposed the lungs to a limited amount of allergen and only for a limited amount of time. Similarly, none of the subjects exhibited lymphadenopathy, which may be related to the timing of the exam (within 10 hours of the challenge) or related to the amount of instilled allergen, which may not be large enough to elicit a more robust, systemic immune response.
There is limited data regarding the expected imaging appearance of the lung following BAL. Gurney et al. described the findings on a plain radiograph in 1987 following planned BAL and serial radiographs and reported “opacities”, and “consolidations” in the lobes lavaged (32). Animal studies have reported increased interstitial opacities on radiographs following lavage (33). Finally, BAL has also been previously reported as a potential cause of increased FDG uptake in the lungs (34). To our knowledge, no study has described the airway and parenchymal changes on CT following BAL in the general population or in those with atopy.
In our cohort, approximately three-quarters of the subjects had ground-glass opacities in the segments wherein the BAL was performed (Figure 2). These findings likely reflect residual lavage fluid in the alveoli rather than cellular infiltrate or inflammation. It has been reported that approximately 50% of the lavage fluid instilled remain in the lungs after aspiration and that the degree of imaging abnormalities correlated well with the amount of retained lavage fluid (32). Our finding of ground-glass opacities rather than more dense consolidations as reported by Gurney et al. is likely related to the timing of the imaging exam (32). While in the previous study, imaging was performed immediately (within 5 minutes) of the BAL and then sequentially over the next 24 hours, we imaged approximately 10 hours after the BAL. This time likely allowed for the resorption of the fluid, consistent with the gradual reduction in opacity over 24 hours previously reported (32). A few subjects, both asthmatics, and non-asthmatics also had consolidations (3/20), nodule formation (4/20), and mild bronchial wall thickening (2/20). We suspect that these findings may be post-traumatic in etiology, related to the relatively rapid instillation and aspiration of the lavage fluid. The procedure may have also resulted in distal bronchial impaction resulting in apparent nodular formation.
Recognizing the normal appearance following BAL is important so as not to be confused with significant pathology (34). While infrequent, CT may be performed following BAL if there are potential complications such as bleeding following the procedure. Ground-glass opacities may be expected in these cases and should not be confused with pathology. In addition, if both imaging and BAL are planned on a patient, it may be prudent to obtain the imaging prior to BAL or to defer imaging at least 24 hours after BAL to minimize confounding findings on CT.
Our study is limited by the small sample size. We also only compared asthmatics and non-asthmatics subjects, all of whom were atopic. These may limit the findings’ generalizability. The findings were also in the setting of a very local instillation of allergen, and it is unclear how these findings would translate in the clinical setting, where allergen is typically distributed more widely in the lungs. Finally, we had no long term follow up imaging to determine if and when the abnormalities and changes noted resolve over time. Nevertheless, our findings may have important clinical implications in the assessment of atopic patients, especially asthmatics, who present with an exacerbation and in those who undergo BAL.
Conclusions
In conclusion, acute allergic response in the lungs can result in significant bronchial wall thickening, formation of consolidative opacities, and development of interstitial septal thickening in atopic patients. Consolidations and severe bronchial wall thickening may be particularly common in asthmatics even without infection should be interpreted with caution when encountered on imaging in atopic patients to prevent misdiagnosis. Additionally, localized ground-glass opacities may be expected up to 10 hours on CT following routine BAL, and care should be taken so as to not misinterpret these as significant pathology.
Acknowledgments
Funding: This work was supported by grants to Andrew D. Luster NIH U19AI095261, R37AI040618, and T32HL116275; Josalyn Cho K08AI113083.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1719
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1719
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1719). JWN reports other from Genentech and Boehringer-Ingelheim, outside the submitted work. SRD reports other from Merck, Pfizer, Bristol Mayer Squibb, Novartis, Roche, Polaris, Cascadian, Abbvie, Gradalis, Clinical Bay, Zai laboratories, and Siemens, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work (including full data access, integrity of the data and the accuracy of the data analysis) in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by institutional review board of Partners Healthcare (IRB2008P000408). Informed consent was taken from all the patients
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Croisant S. Epidemiology of asthma: prevalence and burden of disease. Adv Exp Med Biol 2014;795:17-29. [Crossref] [PubMed]
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008;31:143-78. [Crossref] [PubMed]
- National Asthma Education and Prevention Program. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma-summary report. J Allergy Clin Immunol 2007;120:S94-138. [PubMed]
- Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622-39. [Crossref] [PubMed]
- Harris RS, Venegas JG, Wongviriyawong C, et al. 18F-FDG uptake rate is a biomarker of eosinophilic inflammation and airway response in asthma. J Nucl Med 2011;52:1713-20. [Crossref] [PubMed]
- Taylor IK, Hill AA, Hayes M, et al. Imaging allergen-invoked airway inflammation in atopic asthma with [18F]-fluorodeoxyglucose and positron emission tomography. Lancet 1996;347:937-40. [Crossref] [PubMed]
- Kelly VJ, Winkler T, Venegas JG, et al. Allergic Non-Asthmatic Adults Have Regional Pulmonary Responses to Segmental Allergen Challenge. PLoS One 2015;10:e0143976. [Crossref] [PubMed]
- Harris RS, Winkler T, Tgavalekos N, et al. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am J Respir Crit Care Med 2006;174:245-53. [Crossref] [PubMed]
- Adams DC, Miller AJ, Applegate MB, et al. Quantitative assessment of airway remodelling and response to allergen in asthma. Respirology 2019;24:1073-80. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Lynch DA, Newell JD, Tschomper BA, et al. Uncomplicated asthma in adults: comparison of CT appearance of the lungs in asthmatic and healthy subjects. Radiology 1993;188:829-33. [Crossref] [PubMed]
- Bogaert P, Tournoy KG, Naessens T, et al. Where asthma and hypersensitivity pneumonitis meet and differ: noneosinophilic severe asthma. Am J Pathol 2009;174:3-13. [Crossref] [PubMed]
- Niimi A, Matsumoto H, Takemura M, et al. Clinical assessment of airway remodeling in asthma: utility of computed tomography. Clin Rev Allergy Immunol 2004;27:45-58. [Crossref] [PubMed]
- Niimi A, Matsumoto H, Amitani R, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med 2000;162:1518-23. [Crossref] [PubMed]
- Awadh N, Müller NL, Park CS, et al. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax 1998;53:248-53. [Crossref] [PubMed]
- Svenningsen S, Haider E, Boylan C, et al. CT and Functional MRI to Evaluate Airway Mucus in Severe Asthma. Chest 2019;155:1178-89. [Crossref] [PubMed]
- Jiang D, Wang Z, Shen C, et al. Small airway dysfunction may be an indicator of early asthma: findings from high-resolution CT. Ann Allergy Asthma Immunol 2019;122:498-501. [Crossref] [PubMed]
- Saetta M, Turato G. Airway pathology in asthma. European Respiratory Journal 2001;34:18s-23s. [Crossref] [PubMed]
- Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res 2017;367:551-69. [Crossref] [PubMed]
- Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 2013;144:1026-32. [Crossref] [PubMed]
- Cho JL, Ling MF, Adams DC, et al. Allergic asthma is distinguished by sensitivity of allergen-specific CD4+ T cells and airway structural cells to type 2 inflammation. Sci Transl Med 2016;8:359ra132. [Crossref] [PubMed]
- James A, Mauad T, Abramson M, et al. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med 2012;186:568. [Crossref] [PubMed]
- Bara I, Ozier A, Tunon de Lara JM, et al. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J 2010;36:1174-84. [Crossref] [PubMed]
- Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095. [Crossref] [PubMed]
- Roche WR, Williams J, Beasley R, et al. Subepithelial fibrosis in the beonchi of asthmatics. Lancet 1989;1:520-4. [Crossref] [PubMed]
- Okumura S, Sagara H, Fukuda T, et al. FcepsilonRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol 2005;115:272-9. [Crossref] [PubMed]
- Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol 2006;117:1277-84. [Crossref] [PubMed]
- Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779-88. [Crossref] [PubMed]
- Roach DJ, Ruangnapa K, Fleck RJ, et al. Structural lung abnormalities on computed tomography correlate with asthma inflammation in bronchoscopic alveolar lavage fluid. J Asthma 2019;12:1-12. [Crossref] [PubMed]
- Dimakou K, Gousiou A, Toumbis M, et al. Investigation of bronchiectasis in severe uncontrolled asthma. Clin Respir J 2018;12:1212-8. [Crossref] [PubMed]
- Coman I, Pola-Bibián B, Barranco P, et al. Bronchiectasis in severe asthma: Clinical features and outcomes. Ann Allergy Asthma Immunol 2018;120:409-13. [Crossref] [PubMed]
- Gurney JW, Harrison WC, Sears K, et al. Bronchoalveolar lavage: radiographic manifestations. Radiology 1987;163:71-4. [Crossref] [PubMed]
- Barton AK, Schulze T, Doherr MG, et al. Influence of bronchoalveolar lavage on thoracic radiography in the horse. J Vet Sci 2018;19:563-9. [Crossref] [PubMed]
- Leong S, O’Connor OJ, Doyle D, et al. Increased fluoro-deoxy-D-glucose uptake on positron emission tomography-computed tomography postbronchoalveolar lavage: a potential cause of radiologic misinterpretation. J Thorac Imaging 2011;26:W89-91. [Crossref] [PubMed]


