
LncRNA MACC1-AS1 attenuates microvascular endothelial cell injury and promotes angiogenesis under hypoxic conditions via modulating miR-6867-5p/TWIST1 in human brain microvascular endothelial cells
Introduction
Cerebrovascular diseases, as the name implies, are mainly associated with vasculopathy that mainly manifests as vascular bleeding and vascular occlusion (1). Transient ischemic attack, ischemic stroke, and hemorrhagic stroke are the common forms of cerebrovascular diseases. Of these, ischemic stroke, which accounts for over 80% of cerebrovascular diseases, remains a leading cause of disability and mortality (2). Currently, the common treatment for vascular occlusion in ischemic stroke is prompt thrombolytic therapy which consists of intravenous thrombolysis and mechanical thrombolysis (3,4). However, due to the therapeutic window of ischemic stroke being narrow, many patients in China do not receive thrombolytic treatment in time. Many studies have explored the mechanism and new drug treatments of ischemia-reperfusion processes (5-8), but further efforts are required to develop mature methods for attenuating the cascade of injuries that occur in stroke.
The main pathophysiological basis of ischemic stroke is ischemic-hypoxia–induced injury (9), with angiogenesis having being confirmed as the vital compensatory mechanism in ischemic stroke (10,11). Thus, it would beneficial to find the key regulator of angiogenesis in ischemic stroke.
Long non-coding RNAs (lncRNAs) are a class of RNA with over 200 nucleotides, and increasing evidence has revealed that lncRNA is aberrantly expressed in patients with ischemic stroke (12,13). LncRNA plays crucial roles in inflammation, cell apoptosis, angiogenesis, and cell proliferation in ischemic stroke. For instance, LncRNA Nespas is reported to have antiapoptotic and anti-inflammatory roles in mice with ischemic stroke (14), LncRNA XIST has been implicated in angiogenesis stimulated by hypoxia (15) and LncRNA DANCR was found to promote cell proliferation and angiogenesis via modulating miR-33a-5p/XBP1s (16). Furthermore, lncRNA MACC1-AS1 has been reported to participate in various cancers (17-19), and was found to be aberrantly expressed in cell models with induced with hypoxia. However, the effects of LncRNA MACC1-AS1 in an ischemic stroke model induced with anoxia remain unknown. TWIST1 is speculated to be the target gene of miR-6867-5p, which is closely related to vascular diseases (20), and we thus predicted miR-6867-5p to be the target of lncRNA MACC1-AS1. Consequently, the current study evaluated effects of lncRNA MACC1-AS1 and its underlying miR-6867-5p/TWIST1 downstream mechanism in a hypoxia-induced cell model. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4915).
Methods
Cell culture and treatment
Human brain microvascular endothelial cells (HBMECs) (Catalog #1000, ScienCell) (1×105 cells/well) were cultured in the EBM-2 medium (Lonza, UK) containing 40 µg/mL endothelial growth supplement (ECGS) and 10% fetal bovine serum (FBS; ATCC® 30-2020™). For the cells in the control group, the HBMECs were cultured in an incubator containing 5% CO2 and 95% air in a humidified condition at 37 °C for 24 hours. For the cell model group (hypoxia treatment), the cells were cultured in the hypoxic atmosphere (1% O2, 5% CO2, and 94% N2) for 24 hours. Cells were preserved in serum-free cell freezing medium (Teyebio, Shanghai, China). The study was approved by China-Japan Union Hospital of Jilin University (No. R3021).
Cell transfection
The HBMECs (1×105 cells/well) were cultured under the same conditions as described above. Then, HBMECs were transfected with pcDNA3.1-MACC1-AS1 and its corresponding empty vector, miR-6867-5p inhibitor, and its negative control (inhibitor NC). The products mentioned above were all procured from the GenePharma Company. Then, transfection was performed via using Tenfect transfection reagent (Teyebio, Shanghai, China) in accordance with the manufacturer’s instructions.
Measurement of oxidative stress level
Reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were used as indicators of oxidative stress and detected using their respective kits. The whole detection process was conducted according to the corresponding manufacturer’s protocols.
Assessment of cell apoptosis
Flow cytometry assay was performed for assessment of cell apoptosis using Annexin V-FITC Apoptosis Detection Kit (Invitrogen) according to the manufacturer’s instructions. Briefly, after treatment in the study groups, the cells were lysed with trypsin and washed. After centrifugation, the cells were harvested and suspended in binding buffer. Then, Annexin V-FITC (5 µL) and propidium iodide (5 µL) were added into the binding buffer in succession and incubated with the cells in the dark. The cell apoptosis analysis was performed with flow cytometry (Beckman Coulter Inc.).
Transwell migration assay
After transfection in the different study groups, the HBMECs were resuspended in the upper chamber with serum-free medium (200 µL), and the final cell density was 1×105 cells/well. The lower chamber was filled with 10% FBS-containing medium (600 µL) which served as the chemotactic factor. After HBMECs were incubated under hypoxic conditions for 24 hours at 37 °C, the cells were fixed with formaldehyde. Then, 0.5% crystal violet was added to stain the cells for half an hour. The cell migration was calculated under the microscope.
Clone formation assay
The cell proliferation in the study group was elevated via clone formation assay. After transfection in the study group, the HBMECs were harvested using 0.25% trypsin when they were in the logarithmic phase. The collected cells (1×103) were seeded in the six-well plate in normal HBMEC medium and incubated under the hypoxic conditions described above. The cell culture medium was changed every other day. After 2 weeks of incubation, the cells were washed with phosphate-buffered saline (PBS). Subsequently, methyl alcohol (2 mL) was added to fix the HBMECs, and 0.1% crystal violet was added to stain the cells at room temperature for half an hour. Then, the cells in the different groups were counted and photographed.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The isolation of total RNA was performed via using TRIzol reagents (Thermo Fisher Scientific, Inc.) following the protocol of the manufacturer. Reverse transcription was conducted using PrimeScript RT reagent Kit (Takara). The RT-qPCR assay was conducted on ABI7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) under the following condition: 95 °C for 5 minutes as the condition of initial denaturation; and 40 cycles of 95 °C for 15 seconds, 60 °C for 30 seconds, and 72 °C for 30 seconds. The primers were as follows: MACC1-AS1: 5'-GCCAGTCAGAAAATGAGGAAC-3' (forward), 5'-CCAGTTGGGTGAACAGGAC-3' (reverse); MiR-6867-5p: 5'-CGGTGTGTGTGTAGAGGAAGAA-3' (forward), 5'-GAGGGAGAGGCAGTCAGGTT-3' (reverse); TWIST1: 5'-ACGAGCTGGACTCCAAGATG-3' (forward), 5'-CACGCCCTGTTTCTTTGAAT-3' (reverse). GAPDH and U6 served as the internal control. Relative transcripts expression levels were calculated using the 2−△△ct method.
Tube formation assay
The transfected HBMECs were collected and adjusted to 1×104 cells/well when cell monolayers reached 90% confluency. The cells were then seeded into Matrigel-coated 96-well plates. Hypoxia treatment was performed in the study group (except for control group) as described above. Tube formation was observed and calculated under an Olympus IX70 microscope (Tokyo, Japan).
Western blot
The cells in the study group were lysed by total protein extraction kit (Teyebio, Shanghai, China), and total protein samples were extracted. BCA protein assay kit (Teyebio, Shanghai, China) was used to detect the concentration of proteins. The separation of the proteins was performed on 15% SDS-PAGE gels, and then the resultants were blotted on polyvinylidene difluoride membranes which were further blocked with 5% nonfat milk (room temperature, 1 hour). Subsequently, the membranes were reacted with the primary antibodies against Twist1 (#ab50581, Abcam) and VE-cadherin (#ab232880, Abcam) at 4 °C, overnight. Next, the secondary antibodies (#ab150081, Abcam) were incubated with the membranes. The bands were visualized using an enhanced chemiluminescence kit (Teyebio, Shanghai, China). ImageJ software (Version 1.46; National Institutes of Health) was used for analysis of the gray value.
Assessment of cell barrier function
The HBMECs after transfection were seeded in the Transwell chamber in the incubator at a density of 1×105 cells/cm2 and at 37 °C. After hypoxic treatment, the original medium was removed and phenol red free medium containing fluorescein isothiocyanate (FITC)-labeled dextran (0.5 mg/mL) was added into the upper chamber. After incubation for 1 hour in the incubator at 37 °C, fluorescence intensity was detected using Synergy H2 Microplate Reader (Bio Tek Instruments), and the permeability of the FITC-labeled dextran was calculated based on this measurement.
Luciferase reporter assay
Based on bioinformatics tools, miR-6867-5p was predicted as the target of lncRNA MACC1-AS1, and TWIST1 was predicted as the target of miR-6867-5p. The wild-type binding sites of lncRNA MACC1-AS1 and TWIST1 were inserted into the pmirGLO Dual-luciferase miRNA Target Expression Vector (Promega). The mutated binding site of lncRNA MACC1-AS1/TWIST1 was generated using Q5 Site Directed Mutagenesis Kit (New England Biolabs). Then, wild or mutated lncRNA MACC1-AS1/TWIST1 was co-transfected with miR-6867-5p mimic/NC into the HEK-293T cells. After transfection for 48 hours, the cells were harvested, and the luciferase activity was measured using Dual-Luciferase Reporter Gene Assay Kit (Beyotime, Shanghai, China) on a luciferase reporter system (Promega).
RNA pull down assay
The biotinylated mutant or wild type miR-6867-5p or its negative control (NC-bio) was transfected into the cells. The cells were lysed using lysis buffer at 48 hours post-transfection. Then, streptavidin agarose beads (Invitrogen) were incubated with the cell lysates, and the RNA complex that bound to the beads was eluted and purified using TRIzol (Takara). qRT-PCR was performed to analyze the gene level in the RNA complex.
Statistical analysis
The data analysis was performed with SPSS (Version of 11.0) and GraphPad Prism 6. The data are presented as means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for significant difference analysis. P value <0.05 indicated statistical difference.
Results
LncRNA MACC1-AS1 was downregulated in hypoxia-induced vascular endothelial cells, while lncRNA MACC1-AS1 overexpression was associated with anti-apoptosis and pro-proliferation effects in hypoxia-induced vascular endothelial cells
In order to investigate the potential role of lncRNA MACC1-AS1 in hypoxia-induced injuries, a cell model was established via hypoxia treatment. lncRNA MACC1-AS1 level was detected via RT-qPCR assay. We found that the lncRNA MACC1-AS1 level was decreased significantly in the cell model group, suggesting that lncRNA MACC1-AS1 may play a certain role in hypoxia-induced vascular endothelial cells (Figure 1A). In order to further explore the biological role of lncRNA MACC1-AS1, we investigated the effects of lncRNA MACC1-AS1 overexpression in hypoxia-induced cells. The lncRNA MACC1-AS1 level was highest in the lncRNA MACC1-AS1 overexpression group among all the study groups (Figure 1A) indicating that lncRNA MACC1-AS1 overexpression was obtained successfully. The cell apoptosis and proliferation, as vital indicators of cell injury, were also assessed. In the cell model group, cell apoptosis was significantly increased, and cell proliferation, which was indicated by the clone formation capacity, was obviously reduced, demonstrating that the hypoxia-induced cell model was established successfully (Figure 1B,C). The cell apoptosis induced by hypoxia was markedly attenuated by lncRNA MACC1-AS1 overexpression (Figure 1B,C), and simultaneously, clone formation capacity induced by hypoxia was enhanced by lncRNA MACC1-AS1 overexpression (Figure 1D,E), confirming that lncRNA MACC1-AS1 overexpression has protective effects on hypoxia-induced injuries via reducing cell apoptosis and promoting cell proliferation.
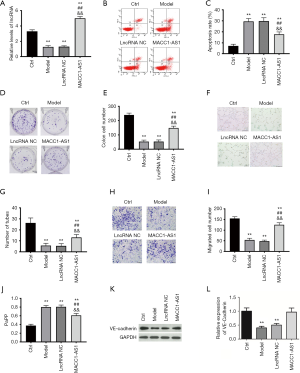
LncRNA MACC1-AS1 overexpression inhibited oxidative stress, promoted cell migration and tube formation, and maintained cell barrier function under hypoxic conditions.
Oxidative stress is a vital parameter in hypoxia-induced injuries. In the current research, ROS, MDA, SOD, and CAT as the indicators of oxidative stress, were detected. There was a significant increase in the levels of oxidation products including ROS and MDA in the model group (Table 1). Meanwhile, the anti-oxidant agents, including SOD and CAT, were significantly reduced in the model group. The results mentioned above confirmed that oxidative stress was enhanced by hypoxia treatment. In the lncRNA MACC1-AS1 overexpression group, the levels of ROS, MDA, SOD, and CAT induced by hypoxia were reduced, revealing that LncRNA MACC1-AS1 overexpression exerted protective effects via inhibiting oxidative stress under hypoxic conditions.

Full table
Angiogenesis is a vital compensatory mechanism, and tube formation assay was further performed to evaluate the effects of LncRNA MACC1-AS1 overexpression on angiogenesis under hypoxic conditions. A significant decrease was found in tube formation level in the model group when compared with the control group (Figure 1F,G). After LncRNA MACC1-AS1 overexpression, the tube formation capacity was enhanced under hypoxic conditions.
Cell migration, as one the processes in angiogenesis, was also evaluated. It was observed that cell migration was inhibited under hypoxic conditions in comparison with the control group (Figure 1H,I). The cell migration level induced by hypoxia was further increased by LncRNA MACC1-AS1 overexpression. Cell barrier function was also evaluated via detecting permeability and VE-cadherin. Cell barrier function was injured in the model group, which was reflected in the increased permeability and reduced VE-cadherin level in this group (Figure 1J,K). Meanwhile, LncRNA MACC1-AS1 overexpression under hypoxic conditions led to a decrease in permeability and an increase in VE-cadherin level. Overall, lncRNA MACC1-AS1 overexpression exerted anti-oxidative stress effects, contributed to cell migration, and enhanced cell barrier function and angiogenesis under hypoxic conditions.
MiR-6867-5p is the downstream target of lncRNA MACC1-AS1
In order to explore the mechanism by which the effects of lncRNA MACC1-AS1 were realized under hypoxic conditions, the candidate target of lncRNA MACC1-AS1 was screened using a bioinformatics online tool. As MiR-6867-5p had the highest score, it was predicted as the target of lncRNA MACC1-AS1 and was further investigated in this study (Figure 2A,B). This target prediction was further validated via luciferase reporter assay. After all the binding sites were investigated, the luciferase activity in the wild type of the lncRNA MACC1-AS1 plus miR-6867-5p mimic group was found to be lower than that in any other study group, revealing that miR-6867-5p was directly targeted by lncRNA MACC1-AS1 (Figure 2C). Moreover, the miR-6867-5p level was increased in the model group compared to the control, and was decreased in the lncRNA MACC1-AS1 overexpression group, which is expected when assuming a targeted silencing relationship between lncRNA MACC1-AS1 and miR-6867-5p (Figure 2D). We validated the regulation relationship between miR-6867-5p and MACC1-AS1 by transfected miR-6867-5p mimic, MACC1-AS1, and both miR-6867-5p mimic and MACC1-AS1 to HBMECs, respectively, followed by miR-6867-5p expression detection, and observed consistent result (Figure 2 E). As shown by the results of the RNA pull-down assay, the lncRNA MACC1-AS1 level was higher in the miRNA-bio group (Figure 2F), further supporting the notion that miR-6867-5p acts as the target of lncRNA MACC1-AS1.
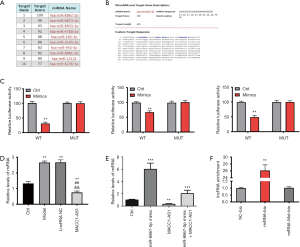
LncRNA MACC1-AS1 functions via regulating miR-6867-5p/TWIST1 in hypoxia-induced vascular endothelial cells
As stated above, the overexpression of lncRNA MACC1-AS1, which could downregulate miR-6867-5p, exerted protective effects under hypoxic conditions. To further investigate whether lncRNA MACC1-AS1 overexpression functions via sponging miR-6867-5p, the effects of miR-6867-5p downregulation were explored in hypoxia-induced vascular endothelial cells. Results showed that miR-6867-5p level was increased in the model group when compared with the control (Figure 3A), which is consistent with the results in Figure 2D. Furthermore, miR-6867-5p level reduced significantly following miR-6867-5p inhibitor treatment. Consistent with the above-mentioned results (Figure 1), increased cell apoptosis, declined clone formation capacity, decreased cell migration, reduced tube formation, increased permeability, declined VE-cadherin level, and elevated oxidative stress level were found in the model group when compared with the control (Figure 3A,B,C,D,E,F,G,H,I,J,K and Table 2). The cell proliferation, migration, and tube formation level under hypoxic conditions were all enhanced, while cell apoptosis and oxidative stress were reduced by miR-6867-5p inhibitor. The effects of miR-6867-5p downregulation are the same with the role of lncRNA MACC1-AS1 overexpression in hypoxia-induced vascular endothelial cells, strong support the notion that lncRNA MACC1-AS1 functions via sponging miR-6867-5p under hypoxic conditions.
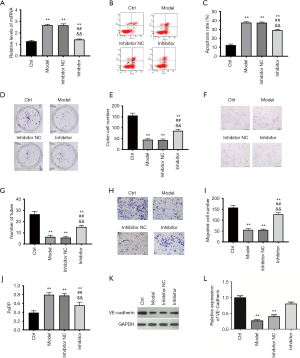

Full table
The downstream target of miR-6867-5p was also investigated. As seen from the results predicted by Targets can, TWIST1 was the target of miR-6867-5p (Figure 4A). Moreover, as presented by the luciferase reporter assay of the four binding sites, a significant decrease was found in luciferase activity of the WT-mimic group, while the luciferase activity remained unchanged in other group (Figure 4B,C,D,E), confirming that TWIST1 is targeted by miR-6867-5p. Moreover, it was observed in the RNA pull-down assay that the TWIST1 level was increased in the miRNA-bio group and unchanged in the other groups (Figure 4F), further indicating that TWIST1 is the direct target of miR-6867-5p. In order to further explore the relationship among lncRNA MACC1-AS1, miR-6867-5p, and TWIST1, we also conducted Western blot assay. We found that TWIST1 level was reduced significantly in the model group and markedly increased in the lncRNA MACC1-AS1 overexpression group and miR-6867-5p inhibitor group (Figure 4G,H). Since lncRNA MACC1-AS1 overexpression resulted in miR-6867-5p downregulation which could further upregulate the TWIST1 level, the results of Western blot assay were consistent with there being a regulatory relationship across the LncRNA MACC1-AS1–miR-6867-5p–TWIST1 axis. All the results provide evidence that LncRNA MACC1-AS1 functions via regulating miR-6867-5p and TWIST1 under hypoxic conditions.
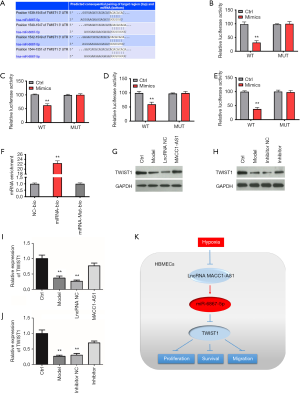
Discussion
The brain is a high-energy consumption organ, and massive amounts of ATP are needed to fuel its various functions (21). Thus, the brain is susceptible to undersupply of essential metabolic substances and oxygen, which can be caused by ischemic stroke. Ischemic stroke is defined by a prolonged interruption of blood flow, and rapid hypoxia may occur during ischemic stroke (22). The under supply of oxygen will cause further vasculopathy and a cascade of injuries (23,24). Vasculopathy acts as prelude to ischemic stroke and is a vital event in ischemic stroke (24,25). Moreover, angiogenesis is a vital compensatory mechanism for ischemic stroke, and provides a possible approach for therapy in ischemic stroke (11). Thus, in this study, we successfully established a hypoxia-induced vascular endothelial cell model as reflected by the increased cell apoptosis, decreased cell proliferation, elevated oxidative stress, reduced angiogenesis capacity, and cell barrier dysfunction.
Recently, increasing evidence has shown that lncRNAs play a pivotal role in ischemic stroke. LncRNA-1810034E14Rik was found to have inhibitory effects on microglia activation in ischemic stroke (26), and LncRNA MEG3 contributed to ischemic stroke by sponging miR-424-5p (27). Some lncRNAs were confirmed to be aberrantly expressed and to participate in the pathophysiology of ischemic stroke (12,13), and in the present study, lncRNA MACC1-AS1 was found to be downregulated in hypoxia-induced vascular endothelial cells. LncRNA MACC1-AS1 has been identified as an oncogene in hepatic carcinoma, lung adenocarcinoma, pancreatic carcinoma, gastric cancer, and other cancers (19,28-30), but its role in other diseases remains relatively unexplored.
In cancer, lncRNA MACC1-AS1 works as a cell growth regulator and promotes cell proliferation (31). In the present research, we found that lncRNA MACC1-AS1 overexpression also promoted HBMEC proliferation and reduced cell apoptosis in hypoxia-induced vascular endothelial cells. Cell proliferation is a vital step for angiogenesis (32), and the results herein indicate a restorative and protective role of lncRNA MACC1-AS1 in ischemic stroke. Angiogenesis is involved in multiple biological processes including cell proliferation, cell migration, and tube formation (32,33). Therefore, on order to further explore the pro-angiogenesis role of lncRNA MACC1-AS1 in hypoxia-induced vascular endothelial cells, tube formation capacity and cell migration were also investigated. We found that tube formation and cell migration were all enhanced by lncRNA MACC1-AS1 overexpression, strongly supporting the notion that lncRNA MACC1-AS1 has a pro-angiogenesis role under hypoxic conditions.
The blood-brain barrier is crucial for maintaining a homeostatic microenvironment. BMECs are the main components of the blood-brain barrier and are essential for keeping the blood-brain barrier intact (34). During ischemic stroke, the blood-brain barrier is disrupted, leading to a series of injuries. In this study, the cell barrier function was disrupted under hypoxic conditions, as indicated by the enhanced permeability and loss of a vital substance, VE-cadherin, which maintains cell barrier function. Meanwhile, the overexpression of lncRNA MACC1-AS1 contributed to keeping the cell barrier function intact.
Oxidative stress is an important event in ischemic stroke and oxidative stress after ischemic stroke results in a series of injuries to all cell components (35). In the current study, we found that the overexpression of lncRNA MACC1-AS1 could attenuate oxidative stress via reducing ROS and MDA and elevating anti-oxidant agents (SOD and CAT).
We also investigated the mechanism underlying the effect of lncRNA MACC1-AS1 in hypoxic conditions. As lncRNA always functions via the regulation of microRNA (miRNA), we screened the target of lncRNA MACC1-AS1. Our data revealed that miR-6867-5p was directly targeted by lncRNA MACC1-AS1. MiR-6867-5p is a newly discovered miRNA, and has received little research attention thus far. In a previous study, proliferation of endometriosis was found to be suppressed by quercetin via inducing miR-6867-5p and some other miRNAs (36). The present study was first to investigate miR-6867-5p in hypoxia-induced vascular endothelial cells. In order to determine whether the role of lncRNA MACC1-AS1 overexpression is effectuated via sponging miR-6867-5p, the effects of miR-6867-5p inhibitor were investigated under hypoxic conditions. As expected, the effects of lncRNA MACC1-AS1 overexpression on cell apoptosis, proliferation, tube formation, angiogenesis, and cell barrier function were consistent with those of miR-6867-5p inhibitor, strongly demonstrating that lncRNA MACC1-AS1 functions via sponging miR-6867-5p.
Since miRNA is a vital regulator in many physiological and pathological processes via targeting certain genes, the target of miR-6867-5p was also explored. As seen from the prediction of bioinformatics tools, the validation of luciferase reporter assay, and RNA pull-down assay, TWIST1 was confirmed as the target of miR-6867-5p. TWIST1 has been demonstrated to act as a promoting factor in the angiogenesis of physiological and pathological processes of a variety of organs (37-40). The pro-angiogenesis role of lncRNA MACC1-AS may function via the upregulation of TWIST1 through sponging miR-6867-5p. In our study, TWIST1 was first investigated in hypoxia-induced human vascular endothelial cells. We found that in comparison with the control, TWIST1 was downregulated in the model group and elevated in the miR-6867-5p inhibitor and lncRNA MACC1-AS1 overexpression group. This provides evidence for the existence of an lncRNA MACC1-AS1–miR-6867-5p–TWIST1 regulatory axis, and further points to the regulation of miR-6867-5p/TWIST1 as the mechanism by which lncRNA MACC1-AS1 functions in hypoxia-induced vascular endothelial cells.
Conclusions
In the present study, we discovered a new function of lncRNA MACC1-AS1 in hypoxia-induced vascular endothelial cells and investigated its possible mechanism. Our data fully confirmed that lncRNA MACC1-AS1 has protective effects of anti-apoptosis, pro-angiogenesis, maintenance of cell barrier function, and reduction of anti-oxidative stress in HBMECs under hypoxic conditions via regulating miR-6867-5p/TWIST1. lncRNA MACC1-AS1 may thus be a promising therapeutic target. The findings herein pave the way for further study and provide a new strategy for future research on ischemic stroke therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4915
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4915
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4915). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by China-Japan Union Hospital of Jilin University (No. R3021).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferrer I, Vidal N. Neuropathology of cerebrovascular diseases. Handb Clin Neurol 2017;145:79-114. [Crossref] [PubMed]
- Girotra T, Feng W. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): are neurologists feeling more comfortable to RESTART antiplatelet? Ann Transl Med 2019;7:S214. [Crossref] [PubMed]
- Cooray C, Mazya M, Mikulik R, et al. Safety and Outcome of Intravenous Thrombolysis in Stroke Patients on Prophylactic Doses of Low Molecular Weight Heparins at Stroke Onset. Stroke 2019;50:1149-55. [Crossref] [PubMed]
- Fan L, Zang L, Liu X, et al. Outcomes of mechanical thrombectomy with pre-intravenous thrombolysis: a systematic review and meta-analysis. J Neurol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Chai Z, Gong J, Zheng P, et al. Inhibition of miR-19a-3p decreases cerebral ischemia/reperfusion injury by targeting IGFBP3 in vivo and in vitro. Biol Res 2020;53:17. [Crossref] [PubMed]
- Lu X, Dong J, Zheng D, et al. Reperfusion combined with intraarterial administration of resveratrol-loaded nanoparticles improved cerebral ischemia-reperfusion injury in rats. Nanomedicine 2020;28:102208. [Crossref] [PubMed]
- Mu Q, Zhou H, Xu Y, et al. NPD1 inhibits excessive autophagy by targeting RNF146 and wnt/beta-catenin pathway in cerebral ischemia-reperfusion injury. J Recept Signal Transduct Res 2020.1-8. [Crossref] [PubMed]
- Shehata AHF, Ahmed AF, Abdelrehim AB, et al. The impact of single and combined PPAR-alpha and PPAR-gamma activation on the neurological outcomes following cerebral ischemia reperfusion. Life Sci 2020;252:117679. [Crossref] [PubMed]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003;4:399-415. [Crossref] [PubMed]
- Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem 2007;102:840-7. [Crossref] [PubMed]
- Li Q, Li Y, Zhang D, et al. Downregulation of microRNA-451 improves cell migration, invasion and tube formation in hypoxia-treated HUVECs by targeting MIF. Mol Med Rep 2019;20:1167-77. [PubMed]
- Dykstra-Aiello C, Jickling GC, Ander BP, et al. Altered Expression of Long Noncoding RNAs in Blood After Ischemic Stroke and Proximity to Putative Stroke Risk Loci. Stroke 2016;47:2896-903. [Crossref] [PubMed]
- Guo X, Yang J, Liang B, et al. Identification of Novel LncRNA Biomarkers and Construction of LncRNA-Related Networks in Han Chinese Patients with Ischemic Stroke. Cell Physiol Biochem 2018;50:2157-75. [Crossref] [PubMed]
- Deng Y, Chen D, Wang L, et al. Silencing of Long Noncoding RNA Nespas Aggravates Microglial Cell Death and Neuroinflammation in Ischemic Stroke. Stroke 2019;50:1850-8. [Crossref] [PubMed]
- Hu C, Bai X, Liu C, et al. Long noncoding RNA XIST participates hypoxia-induced angiogenesis in human brain microvascular endothelial cells through regulating miR-485/SOX7 axis. Am J Transl Res 2019;11:6487-97. [PubMed]
- Zhang M, Tang M, Wu Q, et al. LncRNA DANCR attenuates brain microvascular endothelial cell damage induced by oxygen-glucose deprivation through regulating of miR-33a-5p/XBP1s. Aging (Albany NY) 2020;12:1778-91. [Crossref] [PubMed]
- Chen S, Luo X, Wu W, et al. The long non-coding RNA MACC1-AS1 promotes nasopharyngeal carcinoma cell stemness via suppressing miR-145-mediated inhibition on SMAD2/MACC1-AS1 axis. Biomed Pharmacother 2020;125:109986. [Crossref] [PubMed]
- Jin J, Chen X, Chen J, et al. Long noncoding RNA MACC1-AS1 is a potential sponge of microRNA-34a in cervical squamous cell carcinoma and upregulates cyclin-dependent kinase 6. Oncol Lett 2020;19:2339-45. [PubMed]
- Mao W, Li T. LncRNA MACC1-AS1 Promotes Lung Adenocarcinoma Cell Proliferation by Downregulating PTEN. Cancer Biother Radiopharm 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Nurnberg ST, Guerraty MA, Wirka RC, et al. Genomic profiling of human vascular cells identifies TWIST1 as a causal gene for common vascular diseases. PLoS Genet 2020;16:e1008538. [Crossref] [PubMed]
- Sprick JD, Mallet RT, Przyklenk K, et al. Ischaemic and hypoxic conditioning: potential for protection of vital organs. Exp Physiol 2019;104:278-94. [Crossref] [PubMed]
- Ranjbar Taklimie F, Gasterich N, Scheld M, et al. Hypoxia Induces Astrocyte-Derived Lipocalin-2 in Ischemic Stroke. Int J Mol Sci 2019;20:1271. [Crossref] [PubMed]
- Jayaraj RL, Azimullah S, Beiram R, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation 2019;16:142. [Crossref] [PubMed]
- Hu X, De Silva TM, Chen J, et al. Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ Res 2017;120:449-71. [Crossref] [PubMed]
- Joutel A, Faraci FM. Cerebral small vessel disease: insights and opportunities from mouse models of collagen IV-related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2014;45:1215-21. [Crossref] [PubMed]
- Zhang X, Zhu XL, Ji BY, et al. LncRNA-1810034E14Rik reduces microglia activation in experimental ischemic stroke. J Neuroinflammation 2019;16:75. [Crossref] [PubMed]
- Xiang Y, Zhang Y, Xia Y, et al. LncRNA MEG3 targeting miR-424-5p via MAPK signaling pathway mediates neuronal apoptosis in ischemic stroke. Aging 2020;12:3156-74. [Crossref] [PubMed]
- Guo Y, Zhong J, Wu F, et al. Long noncoding RNA MACC1-AS1 promotes the stemness of hepatocellular carcinoma cells by antagonizing miR-145. J Int Med Res 2020;48:300060520920411. [Crossref] [PubMed]
- Qi C, Xiaofeng C, Dongen L, et al. Long non-coding RNA MACC1-AS1 promoted pancreatic carcinoma progression through activation of PAX8/NOTCH1 signaling pathway. J Exp Clin Cancer Res 2019;38:344. [Crossref] [PubMed]
- Zhao Y, Liu Y, Lin L, et al. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol Cancer 2018;17:69. [Crossref] [PubMed]
- Zhang X, Zhou Y, Chen S, et al. LncRNA MACC1-AS1 sponges multiple miRNAs and RNA-binding protein PTBP1. Oncogenesis 2019;8:73. [Crossref] [PubMed]
- Risau W. Mechanisms of angiogenesis. Nature 1997;386:671-4. [Crossref] [PubMed]
- Ruan L, Wang B. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res 2015;1623:166-73. [Crossref] [PubMed]
- Jiang X, Andjelkovic AV, Zhu L, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 2018;163-164:144-71. [Crossref] [PubMed]
- Zhao H, Han Z, Ji X, et al. Epigenetic Regulation of Oxidative Stress in Ischemic Stroke. Aging Dis 2016;7:295-306. [Crossref] [PubMed]
- Park S, Lim W, Bazer FW, et al. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem 2019;63:87-100. [Crossref] [PubMed]
- Mammoto A, Hendee K, Muyleart M, et al. Endothelial Twist1-PDGFB signaling mediates hypoxia-induced proliferation and migration of alphaSMA-positive cells. Sci Rep 2020;10:7563. [Crossref] [PubMed]
- Mahmoud MM, Kim HR, Xing R, et al. TWIST1 Integrates Endothelial Responses to Flow in Vascular Dysfunction and Atherosclerosis. Circ Res 2016;119:450-62. [Crossref] [PubMed]
- Li J, Liu CH, Sun Y, et al. Endothelial TWIST1 promotes pathological ocular angiogenesis. Invest Ophthalmol Vis Sci 2014;55:8267-77. [Crossref] [PubMed]
- Mammoto T, Jiang A, Jiang E, et al. Role of Twist1 Phosphorylation in Angiogenesis and Pulmonary Fibrosis. Am J Respir Cell Mol Biol 2016;55:633-44. [Crossref] [PubMed]
(English Language Editor: J. Gray)


