
Successful treatment of secondary macrophage activation syndrome with emapalumab in a patient with newly diagnosed adult-onset Still’s disease: case report and review of the literature
Introduction
Macrophage activation syndrome (MAS) is a rare, life-threatening inflammatory disease with 50% mortality stemming from multi-organ failure (1). MAS, also known as hemophagocytic lymphohistiocytosis (HLH), is attributed to excessive activation of the immune system due to increased production of cytokines and enhanced phagocytosis by macrophages in the setting of infection, malignancy or autoimmune disease (2). Primary MAS or HLH is caused by genetic defects limiting the exocytosis of granules and function of cytotoxic T cells as seen in Griscelli syndrome 2 (GS-2) or Chédiak-Higashi syndrome (CHS) (3), which are traditionally treated with bone marrow transplantation (4). Genes mutated in patients with primary or familial MAS are involved in trafficking and docking of the cytolytic granules, including LYST, RAB27A, UNC13D, STXBP2, STX11 (2). In primary MAS, failure to kill the target cell by cytotoxic T lymphocytes (CTL) and natural killer (NK) cells elicits an uncontrolled release of pro-inflammatory cytokines, in particular, IFN-γ (1,5). Life-threatening manifestations of MAS are typically controlled by immediate administration of corticosteroids, etoposide, cyclosporine, or inhibitors of cytokines, such as interleukin-1 (IL-1) antagonist anakinra or interferon-γ-blocking antibody, emapalumab (5,6). Among rheumatic autoimmune diseases, secondary MAS is most commonly associated with systemic juvenile idiopathic arthritis and systemic lupus erythematosus (2). Adult-onset Still’s disease (AOSD) is less commonly associated with MAS, and it is generally treated with inhibitors of tumor necrosis factor α (TNF-α), IL-1, IL-6, or IL-18 (7). Although elevated IFN-γ levels have been reported in AOSD patients (8), this cytokine has not been targeted for therapeutic intervention. Here, we report a 22-year-old female patient with AOSD which was newly diagnosed in the setting of MAS. She had high fevers, anemia, thrombocytopenia, splenomegaly, hematophagocytosis, and elevated serum ferritin (37,950 ng/mL) and CD25 levels (11,870 pg/mL) that remained unresponsive to corticosteroids and anakinra. Her serum IL-1 and TNF-α levels were normal, while IL-6 and interferon gamma (IFN-γ) were elevated. Given the safety (9) and biomarker-driven efficacy of IFN-γ blockade in mouse models (10) and primary MAS patients (11), we initiated emapalumab treatment which was found remarkably effective in eliminating her fevers, arthralgia, and all laboratory abnormalities. This report provides preliminary evidence for therapeutic efficacy for IFN-γ blockade in AOSD and secondary MAS. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3127).
Case presentation
A 22 years old female was transferred to Upstate University Hospital with no significant past medical history other than mild intellectual disability and “knock knees” for further work-up and management of quotidian, remitting, and relapsing fevers, chills, and diffuse pruritic rash that first involved her extremities and subsequently spread to her entire body for two weeks prior to her hospitalization. Prior to her hospital admission, she went to urgent care for a pruritic rash which improved but did not resolve with a 5-day course of prednisone 20 mg/day. The family then presented to an emergency room at an outside hospital for diffuse pruritic rash and fever of 38.9 °C. Basic labs and infectious workup for flu and streptococcus were negative, and she was sent home with acetaminophen, diphenhydramine and another 2-week tapering course of prednisone. Prednisone and diphenhydramine helped dissipate the rash, but she remained febrile. One week later she presented to our hospital with persistent fevers, rash, arthralgia, and myalgia. She denied any weight loss, weakness, headaches, changes in vison, nausea, vomiting, abdominal pain, diarrhea, dysuria, hematuria, mucosal ulcers, photosensitive rash, pleurisy or any other complaints. Her past medical history was remarkable for developmental delay and a 2-year history of intermittent left knee pain secondary to previous trauma. Her medications upon admission included daily acetaminophen, diphenhydramine and prednisone. She had no known allergies, or family history of autoimmune diseases or cancers.
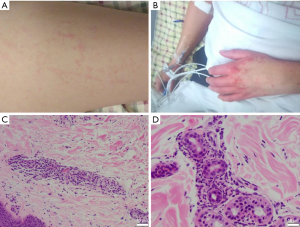
On admission, the patient was in mild distress. The temperature was 39 °C (Figure 1), blood pressure of 122/80 mmHg, pulse of 135 beats per minute, and oxygen saturation of 94% on ambient air. Conjunctivae were pale, but not icteric. Her nasal and oral mucosae were clear. Lymphadenopathy was not palpable. Her liver function tests (LFTs) (Figure 1) and ferritin were elevated (Figure 1). She had a diffuse pinkish, non-blanching and macular rash located bilaterally on cheeks, chest, abdomen, back, buttocks, legs, arms, and hands (Figure 2). Pulmonary exam was normal without crackles or wheezes. The patient had tachycardia but regular rhythm. There was no pericardial friction rub, murmur, or gallop. There was no abdominal tenderness or organomegaly. There was no lower extremity edema. The remainder of the physical and neurological examinations was unremarkable.

Laboratory studies showed leukocyte count of 9,500/µL (normal range: 4,100–11,000/µL; normal range is provided in parentheses for laboratory studies), hemoglobin 12 g/dL (12–16 g/dL), and platelet count 156,000/µL (150,000–450,000/µL). Her erythrocyte sedimentation rate (ESR) was 25 mm/h (0–30 mm/h). Prothombin time international normalized ratio was 1.29. C-reactive protein (CRP) was 84.4 mg/L (<8.0 mg/L). Ferritin was 6,450 ng/mL (13–150 ng/mL) on day 2 after admission. Lactate dehydrogenase was 290 U/L (84–246 U/L). Haptoglobin was 254 mg/dL (30–200 mg/dL). The following laboratory tests were normal on admission: creatinine kinase, 17 U/L (20–180 U/L); blood urea nitrogen, 8 mg/dL (7–24 mg/dL); creatinine, 0.63 mg/dL (0.6–1.0 mg/dL); aspartate aminotransferase, 21 U/L (11–39 U/L); alanine aminotransferase, 8 U/L (12–78 U/L); alkaline phosphatase, 55 U/L (45–117 U/L); albumin, 3.5 g/dL (3.2–4.5 g/dL); total bilirubin, 0.6 mg/dL (0–1.0 mg/dL); rheumatoid factor, 13 units (<14 IU/mL); Sjogren syndrome A autoantibody, 56 (0–99 AU/mL); Sjogren syndrome B autoantibody, 15 (0–99 AU/mL); Smith autoantibody, 10 (0–99 AU/mL); ribonucleoprotein autoantibody, 19 (0–99 AU/mL); scleroderma-70 autoantibody, 25 (0–99 AU/mL); centromere autoantibody, 2 AU/mL (0–99 AU/mL); Jo-1 autoantibody, 9 (0–99 AU/mL); double-stranded DNA antibody, 20 (0–99 AU/mL); histone antibody, 11 (0–99 AU/mL). Anti-nuclear antibody (ANA) and anti-neutrophil cytoplasmic antibody tests were negative. Complements C3 and C4 were normal at 112 mg/dL (90–180 mg/dL) and 18 mg/dL (10–40 mg/dL), respectively, while total complement activity (CH50) was 40 U/mL (42–999,999 U/mL). Soluble interleukin-2 receptor alpha (sIL-2Rα or sCD25) was elevated at 1,372 U/mL (223–710 U/mL). β2 glycoprotein I and cardiolipin IgA, IgG, IgM antibodies and diluted Russell viper venom time, hexagonal phase phospholipid neutralization and platelet neutralization procedures were all negative. Direct and indirect Coombs tests were negative. Urine protein/creatinine ratio was 0.29 mg/mg. Infectious workup included blood cultures and serological testing for human immune deficiency virus-1, syphilis, hepatitis A, B and C viruses, parvovirus B19, Lyme IgM and IgG antibodies, and monospot assay, which were all negative. EBV nuclear antigen antibody was elevated at 2.52 (reference range: <0.91 ISR) and EBV VCA IgG p18 antibody was elevated at 2.93 (<0.91 ISR). However, EBV IgM was within normal limits and EBV DNA PCR was negative. CMV IgM and IgG were negative. Coxsackie A Type 16 IgG titers were elevated at 1:200 (negative: <1:100 titer). Coxsackie A24 IgG titer was elevated at 1:200 (negative: <1:100 titer). Coxsackie A7, A16, and A24 IgM antibodies were negative. This female Caucasian patient had no family history of AOSD or MAS, and genetic testing for mutations in LYST, RAB27A, UNC13D, STXBP2, and STX11 excluded primary MAS (2). CT thorax with contrast showed trace left pleural effusion with minimal atelectasis in the left lung base. CT abdomen/pelvis with contrast showed scattered sub-centimeter retroperitoneal lymph nodes without evidence of organomegaly or masses. A peripheral blood leukemia and lymphoma panel was negative.
Upon admission, she was evaluated by rheumatology. Adult onset stills disease (AOSD) was a considered as a primary diagnosis given her fevers of >39 °C ongoing for 2 weeks, recurrent bilateral knee pain and tenderness, mild leukocytosis, elevated ferritin, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels and diffuse erythematous rash. She met all four major criteria and three of four minor criteria for the diagnosis of AOSD (12), while active infections and malignancy were excluded (Table 1). Punch biopsy of the skin rash was consistent with neutrophilic urticarial dermatosis (Figure 2), which is commonly seen in AOSD (13-15). Three days after admission the decision was made to start prednisone 60 mg/day for AOSD. Despite 60 mg/day prednisone she continued to spike fevers and ferritin continued to increase while ESR and CRP were normalized. There was a concern for MAS, and therefore, ferritin, triglycerides, fibrinogen, CBC with differential and CMP were trended daily. She was pulse dosed daily with 1 g of intravenous (IV) methylprednisolone for 3 days starting on the 4th day of her hospital course. This was followed by 60 mg of daily IV methylprednisolone along with 100 mg of daily subcutaneous anakinra for underlying AOSD. She initially presented with a platelet count of 156,000/uL which down trended to 73,000/uL thirteen days into hospitalization. Her spleen size was 13.8 cm on admission, and it reached 15.4 cm on day 14 of hospitalization (Table 2).

Full table

Full table
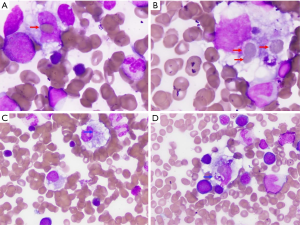
Despite receiving eleven days of intravenous corticosteroids and anakinra, the patient continued to have high fevers and rash and her labs kept worsening (Figure 1). Her ferritin steadily increased peaking at 37,950 ng/mL (13–150 ng/mL). Thus the patient already met four of the eight HLH-2004 criteria (splenomegaly, fever >38.5 °C, ferritin >500 ng/mL, elevated levels of soluble IL-2 receptor CD25, sCD25; Table 2), NK cell activity, and a bone marrow biopsy were obtained on day 17 of hospitalization. While NK activity was normal, the bone marrow biopsy revealed hemophagocytosis, phagocytosis of intact red cells and platelets (Figure 3), thus solidifying the diagnosis of MAS (Table 2).
In order to facilitate early diagnosis and avoid delays in treatment of MAS, a scoring system termed HScore has been developed (18). The HScore is comprised of 9 criteria: 3 clinical, 5 laboratory and 1 histological (18). The H score has been shown to perform better than the HLH-2004 criteria if used at presentation with sensitivity of 90% and specificity of 79% (18). Our patient had an HScore of 233 that corresponds to >98% probability of MAS (Table 3).
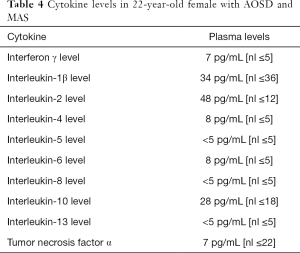
Her serum IL-1 and TNF-α levels were normal, while IL-6 and interferon gamma (IFN-γ) were elevated (Table 4). Molecular studies from the bone marrow did not show mutations in susceptibility genes such as AP3B1, AP3D1, CD27, CD70, CTPS1, GATA2, ITK, LYST, MAGT1, NLRC4, PRF1, RAB27A, SH2D1A, SLC7A7, STX11, STXBP2, UNC13D, XIAP.
Full table
As the patient has persistent fevers and her MAS-relevant laboratory markers have worsened on high dose steroids and IL-1 blockers, she was started on emapalumab at a dosage of 1mg/kg on the 19th day of hospital admission. IV methylprednisolone was switched to IV dexamethasone 20 mg daily. She was also started on acyclovir 400 mg/day for herpes zoster prophylaxis, fluconazole 400 mg/day for fungal prophylaxis, and atovaquone 1,500 mg/day for pneumocystis jiroveci pneumonia prophylaxis while on emapalumab. Febrile episodes recorded as high as 39.4 °C. Repeat abdominal ultrasound showed worsening splenomegaly from 14.5 to 15.4 cm craniocaudal length. Given worsening labs and increasing splenomegaly her emapalumab dosing was increased to 3 mg/kg for her second dose based on emapalumab dosing directions. After the second dose, her ferritin, platelets, LFTs, fibrinogen, and triglycerides all began to improve (Figure 1). Follow up abdominal ultrasound showed improved splenomegaly at 13.6 cm craniocaudal length on day 33 of hospitalization. The patient continued to receive emapalumab at 3 mg/kg and received a total of 6 infusions inpatient. She no longer spiked fevers, was hemodynamically stable, and labs continued to show improvement. She tolerated the infusion well without any side effects or complications. She was discharged on dexamethasone 20 mg/day along with antibiotics for prophylaxis of fungal, viral, and bacterial infections. Her hospital length of stay was 36 days. In the outpatient rheumatology setting, she has since received 3 more infusions of emapalumab and her daily oral dexamethasone dosage has been reduced from 20 mg to 2 mg. Her most recent ferritin from her outpatient office visit was 122 ng/mL. Emapalumab elicited complete resolution of her MAS after five infusions 3 days apart followed by 4 infusions 1 week apart. The last three infusions of 3 mg/kg emapalumab were administered in the outpatient setting. She is currently maintained on 2 mg/day dexamethasone. She is in complete remission with exception of residual rash on her hands (Figure 2).
The patient provided written consent in approval of the publication of her case without personal identifying information. Her consent was witnessed by both of her parents.
Discussion
MAS has an overall mortality rate of 50% (1). Secondary MAS affects up to 15% of AOSD patients (19), and it is considered to be the most severe complication of the disease with high mortality rate approaching 41% (20). Treatment for primary MAS/HLH requires bone marrow transplantation along with immunosuppression using dexamethasone, etoposide, cyclosporine A and intrathecal methotrexate for CNS involvement (21). There has been little consensus on the treatment of secondary MAS. Therapy is typically directed at addressing the MAS and the underlying cause, such as infection, malignancy, or autoimmune disease (2).
In primary MAS, failure to kill the target cell leads to uncontrolled expansion of cytotoxic T lymphocytes and NK cells trigger an uncontrolled activation of macrophages and vast overproduction of cytokines particularly IFN-γ (1,2). Emapalumab, is an interferon-gamma blocking monoclonal antibody that was approved on November 20, 2018 by the FDA for adult and pediatric patients with primary HLH with refractory, recurrent or progressive disease or intolerance with conventional therapy. Our patient was unresponsive to high dose steroids and anakinra targeting her AOSD. While IFN-γ production has been implicated in its pathogenesis (8), blockade of this cytokine has not been targeted for therapeutic intervention in AOSD. Given the rapidly fatal course of MAS and the toxicity associated with conventional cytotoxic treatments, such as cyclophosphamide, etoposide, and calcineurin inhibitors (16,22,23), we elected to administer emapalumab, which has been approved by the FDA for refractory primary MAS on November 20, 2018. She had complete resolution of her MAS on emapalumab, which eliminated her fevers, arthralgia, splenomegaly, liver injury, and all laboratory abnormalities. Therefore, this study provides preliminary evidence for therapeutic efficacy for IFN-γ blockade in AOSD and secondary MAS. These findings warrant further assessment of the clinical efficacy of emapalumab in patients with AOSD and secondary MAS.
Conclusions
This study provides preliminary evidence for therapeutic efficacy for IFN-γ blockade in AOSD and secondary MAS. Therefore, elevated production of IFN-γ should be considered as a targetable biomarkers of disease pathogenesis. This report warrants further assessment of the clinical efficacy of emapalumab in patients with AOSD and secondary MAS.
The patient and her family have expressed great appreciation for the care they received. As of 5/26/2020, the patient is in complete remission without any clinical symptom, such as rash or facial swelling due to exposure to high doses of glucocorticoids. The family considered the effect of this new treatment to be a miracle.
Acknowledgments
Funding: Andras Perl is supported in part by grants AI072648, AI122176, AI141304, and AR068052 from the National Institutes of Health.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3127
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3127). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). All data involved in this study were collected retrospectively and didn’t disclose identity information, which did not require subsequent ethics approval. Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology 2013;2013:605-11. [Crossref] [PubMed]
- Crayne CB, Albeituni S, Nichols KE, et al. The immunology of macrophage activation syndrome. Front Immunol 2019;10:119. [Crossref] [PubMed]
- Griscelli C, Durandy A, Guy-Grand D, et al. A syndrome associating partial albinism and immunodeficiency. Am J Med 1978;65:691-702. [Crossref] [PubMed]
- Virelizier JL, Lagrue A, Durandy A, et al. Reversal of Natural Killer Defect in a Patient with Chediak-Higashi Syndrome after Bone-Marrow Transplantation. N Engl J Med 1982;306:1055-6. [Crossref] [PubMed]
- Henderson LA, Cron RQ. Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Childhood Inflammatory Disorders: Diagnosis and Management. Paediatr Drugs 2020;22:29-44. [Crossref] [PubMed]
- Ravelli A, Davi S, Minoia F, et al. Macrophage Activation Syndrome. Hematol Oncol Clin North Am 2015;29:927-41. [Crossref] [PubMed]
- Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol 2018;14:603-18. [Crossref] [PubMed]
- Hoshino T, Ohta A, Yang D, et al. Elevated serum interleukin 6, interferon-y, and tumor necrosis factor-a levels in patients with adult Still’s disease. J Rheumatol 1998;25:396-8. [PubMed]
- Lounder DT, Bin Q, De Min C, et al. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv 2019;3:47-50. [Crossref] [PubMed]
- Jordan MB, Hildeman D, Kappler J, et al. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004;104:735-43. [Crossref] [PubMed]
- Bracaglia C, de Graaf K, Pires Marafon D, et al. Elevated circulating levels of interferon-g and interferon-g-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis 2017;76:166-72. [Crossref] [PubMed]
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol 1992;19:424-30. [PubMed]
- Qiao J, Zhou S, Li S, et al. Histopathological diagnosis of persistent pruritic eruptions associated with adult-onset Still’s disease. Histopathology 2019;74:759-65. [Crossref] [PubMed]
- Nassereddine H, Fite C, Kottler D, et al. An atypical persistent eruption of adult-onset Still’s disease with neutrophilic urticarial dermatosis-like dermal features: A case report and review of the literature. J Cutan Pathol 2018;45:793-9. [Crossref] [PubMed]
- Lee JYY, Hsu CK, Liu MF, et al. Evanescent and persistent pruritic eruptions of adult-onset still disease: a clinical and pathologic study of 36 patients. Semin Arthritis Rheum 2012;42:317-26. [Crossref] [PubMed]
- Jordan MB, Allen CE, Weitzman S, et al. How I treat hemophagocytic lymphohistiocytosis. Blood 2011;118:4041-52. [Crossref] [PubMed]
- Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124-31. [Crossref] [PubMed]
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the H score, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014;66:2613-20. [Crossref] [PubMed]
- Ruscitti P, Rago C, Breda L, et al. Macrophage activation syndrome in Still’s disease: analysis of clinical characteristics and survival in paediatric and adult patients. Clin Rheumatol 2017;36:2839-45. [Crossref] [PubMed]
- Ruscitti P, Cipriani P, Ciccia F, et al. Prognostic factors of macrophage activation syndrome, at the time of diagnosis, in adult patients affected by autoimmune disease: Analysis of 41 cases collected in 2 rheumatologic centers. Autoimmun Rev 2017;16:16-21. [Crossref] [PubMed]
- Hutchinson M, Tattersall RS, Manson JJ. Haemophagocytic lymphohisticytosis-an underrecognized hyperinflammatory syndrome. Rheumatology (Oxford) 2019;58:vi23-30. [Crossref] [PubMed]
- Alongi A, Naddei R, De Miglio L, et al. Macrophage activation syndrome in pediatrics. Pediatr Allergy Immunol 2020;31:13-5. [Crossref] [PubMed]
- Lorenz G, Schul L, Schraml F, et al. Adult macrophage activation syndrome - haemophagocytic lymphohistiocytosis: ‘of plasma exchange and immunosuppressive escalation strategies’ a single centre reflection. Lupus 2020;29:324-33. [Crossref] [PubMed]



