
Efficacy and safety of transcatheter aortic valve implantation in patients with severe bicuspid aortic stenosis
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease, with morbidity of 1% to 2%; the ratio of male to female patients is 2:1 (1-4). The outcomes of clinical progression in BAV patients include aortic stenosis, aortic regurgitation, aortic dilatation, aortic aneurysm, aortic dissection, thrombosis, and infective endocarditis (5,6). As it is a common congenital malformation, bicuspid aortic stenosis accounts for a substantial proportion of patients with aortic valve stenosis (AS). Regarding the anatomical structure of the two aortic valves due to the abnormal blood flow, irregular thickening of the valve, and asymmetric calcification of the valve often occur in BAV patients with aging. Sievers et al. classified BAV into three major types according to numbers of raphae and the position and function of leaflets (7). However, raphe in BAV is found to be closely correlated with moderate to severe AS. Moreover, in patients with severe AS who might require high-risk surgery or are inoperable, transcatheter aortic valve implantation (TAVI), a minimally invasive operation, has emerged as an alternative therapeutic option; it has reduced mortality by 53% (8,9).
Bicuspid AS are more likely to have aortic dilatation with slightly less elliptical annuli [the ratio of minor/major dimension was larger than in tricuspid aortic valve (TAV) patients] (10), which might lead to paravalvular aortic valve regurgitation (AR) and permanent pacemaker implantation (PPM) after TAVI with higher mortality. Bicuspid AS was therefore regarded as a contraindication of TAVI for a long time (11), and most foreign clinical trials of TAVI excluded Bicuspid AS patients. However, Chinese BAV patients usually undergo valve replacement due to ethnic differences and early-onset; therefore, the proportion of BAV morphology in severe AS patients receiving TAVI was 40–50%, which was much higher than the proportion of 1.6–9.3% in western patients. Furthermore, severe Bicuspid AS patients in China often has a higher Calcium volume, leading to more challenges for TAVI (10). However, the indications of TAVI for AS patients with Bicuspid AS are like those for TAV in the ACC/AHA guidelines (12), in that an annulus perimeter-based guide of the valve size might not be suitable in Bicuspid AS with supra-annular deformity. Therefore, a related study was proposed in China, where a supra-annular structure-based sizing strategy was applied in the TAVI procedure for Bicuspid AS patients.
In conclusion, we conducted this study on therapeutic safety and outcomes of TAVI between Tricuspid AS and Bicuspid AS patients for 1 year, which would offer further data and evidence for widening the indications of TAVI in the Chinese population. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4436).
Methods
Patients characteristics
In this study, 118 consecutive patients with severe AS who underwent TAVI from May 2017 to January 2019 in the department of cardiovascular surgery of Tianjin Chest Hospital and Fuwai hospital were enrolled. Severe AS was defined as aortic valve area ≤1.0 cm2 or indexed for body surface area <0.6 cm2/m2 plus either the mean pressure gradient ≥40 mmHg or the peak velocity ≥4.0 m/s. Symptomatic patients had to have dyspnea, New York Heart Association (NYHA) functional class II or higher, angina pectoris, or cardiac syncope to qualify for the trial. According to the indications of TAVI in the ACC/AHA guidelines, all severe AS patients were prospectively enrolled and assessed as having a moderate to high risk of surgery and Society of Thoracic Surgeons score (STS) (13). Depending on cusp number, patients with aortic valve disease were grouped into tricuspid AS, and Bicuspid AS and the Bicuspid AS patients were further classified on raphe. Also, baseline characteristics of the echocardiography and multislice computer tomography (MSCT) measurements were collected as previously described (10). This study was consistent with the principles of the Helsinki declaration (as revised in 2013) and was approved by the ethics committee of Tianjin Chest Hospital (No: IRB-SOP-016(F)-001-02). Patients voluntarily joined the study, signed an informed consent form.
Device description
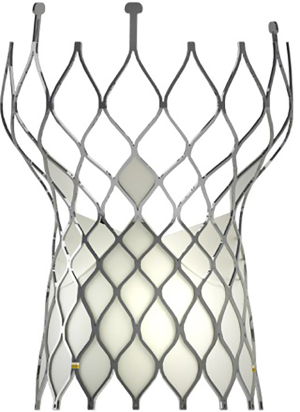
The Venus A-valve, a self-expandable valve, which approved by the China Food and Drug Administration in May 2017, was used in this study. The Venus A-valve was usually placed above the native annular; it has a supra-annular design that reduces the occurrence of valve thrombus (Figure 1). The valve is composed of three porcine pericardial leaflets sutured onto a nitinol alloy stent. First, to correctly position the prosthesis, three radiopaque markers were placed at the bottom of the inflow. Then, the stronger supporting force at the initial 20-mm section of the inflow end was compatible with Bicuspid AS and severe calcification, the two prominent morphology characteristics of Chinese AS patients. Finally, the tapering inlet end of flow could protect the heart from the conduction block. Venus MedTech produces the Venus A-valve in Hangzhou, China, and had been proved as suitable for Chinese patients.
MSCT image analysis
After the injection of contrast agents, data from the MSCT image (100–120 kV) were analyzed respectively for all patients. Curved multiplanar reconstruction (CMPR) analyses were used for annular and aortic valve dimension descriptions (software: 3 mensio ValvesTM, version 5.1; 3mensio Medical Imaging BV, Bilthoven, The Netherlands). Then, the minor and major supra-annular dimension, the ratio of minor/major dimension, perimeter-derived diameter of annulus, and maximal ascending aorta dimension were measured from the ring. Briefly, TAVs annular sizing using the standard of care for the selection of the proper transcatheter heart valve; for Bicuspid AS it has been determined the intercommissural distance 4 or 5 mm above the annular may have supra-annular narrowing size (14), according to the maximum diameter of this plane to select the size of the predilation balloon. Above the level of the virtual annular, we usually measure every 2 mm until the narrowing size plane was determined. The threshold for leaflet calcification detection was set at 850 Hounsfield units (HU), and the Calcium volume was calculated from the left ventricular outflow tract to the leaflet tips.
TAVI procedure
All the procedures were performed in the hybrid operating room with the patients under general anesthesia. Intraprocedural transesophageal echocardiography was performed to assess the performance of the prosthetic valve if there was no contraindication. Usually, transfemoral access is preferred for TAVI, but the trans-ascending aorta approach was used in this study if the patient had anatomical issues with the femoral artery (like smaller diameter, tortuosity, and severe calcification). The candidate prosthesis size of Venus A-valve was comprehensively determined by the operators according to the annular diameter and valve calcification score. Usually, the 23 mm Venus A-valve was designed for a perimeter-derived annulus of 53–63 mm, 26 mm Venus-A-Valve for 63–72 mm, 29 mm Venus-A-Valve for 72–82 mm and 32 mm Venus-A-Valve for 82–91 mm (15). If heavily calcified are present (850 HU >400 mm3) or Bicuspid AS, the heart team, will down the size of to prevent annular rupture.
The aortic balloon valvuloplasty (predilation) was a routine procedure, and the decision not to perform predilation was made primarily in cases with only mild leaflet calcification or with contraindication. The diameter of the prosthesis size was determined according to the final balloon size and perimeter-derived diameter of the annulus; the details have described elsewhere (16) (Figure 2). For Bicuspid AS, the valve was directly implanted in an elevated position, regarding the leaflet extremities rather than the virtual basal annulus, to reduce the risk of paravalvular AR and a permanent pacemaker. Echocardiographic was used to assess the paravalvular AR as [0] none/trace, [1] mild, [2] moderate, and [3] severe to evaluate the AR immediately after the operation. If moderate or severe AR was still present on the dilation of the aorta and added valve was considered. Also, the post-TAVI AR, effective orifice area (EOA), and mean aortic valve gradient (PGmean) were also evaluated at one day for the acute procedural outcome.
Outcomes
Patients were followed at the 1st, 7th, 30th, 180th, and 360th days postoperatively to observe the myocardial functions; the complications were evaluated at post-procedure, 30 days, and 1 year. The myocardial functions were assessed using the New York Heart Association (NYHA) heart failure class and echocardiography, including factors of the AR, EOA, and PGmean. The primary outcomes were the rate of device success post-procedure and all-cause mortality at 1year. The second outcomes included stroke, myocardial infarction, major vascular complications, PPM, acute kidney injury, valve endocarditis, NYHA functional class, and valve performance (as assessed on echocardiography). All outcomes were defined according to Valve Academic Research Consortium-2 definitions (17).
Statistical analysis
SPSS 22.0 software was applied for statistical analysis, and P˂0.05 at two-tailed was considered statistically significant. Measurement data were analyzed using the Shapiro-Wilk test to determine the normal distribution of all variables. Normal distribution data were presented as mean ± standard deviation and compared using the unpaired t-test, ordinal variables were compared using the Mantel-Whitney test. Categorical data were presented as the number (percentage) and compared using the χ2-test or Fisher’s exact test. Cumulative survival rates were analyzed using Kaplan-Meier estimates, and the comparisons were performed with the log-rank test.
Results
Baseline characteristics and procedural statues
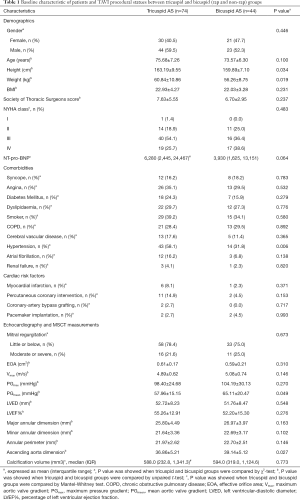
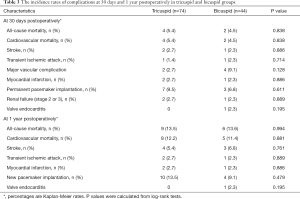
A total of 118 severe AS patients received a TAVI procedure with a self-expandable valve on the supra-annular structure-based sizing strategy were enrolled. The tricuspid AS group with 74 patients and the Bicuspid AS group with 44 patients were followed up at least 1 year. Both groups were well-matched, except for more frequent hypertension in the tricuspid AS group. In the comparative statistical analysis of patient characteristics, ages between the two groups were similar (75.68 vs. 73.57 years). Although the height and weight of patients in both groups had statistically significant differences, the body mass index calculated as weight in kg divided by height in meters squared was nearly equal (22.93 vs. 22.03). As the factors about operative risk, the STS (7.63 vs. 6.70), and NYHA class (at class III–IV) were also well-balanced. The percentage of patients with comorbidities, including syncope, angina, diabetes mellitus, dyslipidemia, smoker, chronic obstructive pulmonary disease, cerebral vascular disease, atrial fibrillation, and renal failure, were analogous without significant difference. Twenty-three point eight percent of patients in both groups had Cardiac risk factors, including myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, pacemaker implantation, and chest-wall irradiation. No patients were lost to follow up.
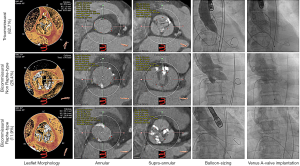
Before the TAVI procedure, echocardiographic and MSCT measurements were collected and analyzed. Due to the distinct anatomic differences between BAV and TAV, the factors related with atretic morphology including major/minor annular dimension (25.80 vs. 26.97 and 21.64 vs. 22.69), annular perimeter (21.97 vs. 22.70), calcium volume (588.0 vs. 594.0) and the factors related with AS severity including mitral regurgitation (mainly with little or below), EOA (0.61 vs. 0.59), maximum velocity (4.89 vs. 5.08), PGmax (98.40 vs. 104.19), left ventricular diastolic diameter (53.72 vs. 51.76). Ejection fraction % (55.26 vs. 52.20), showed some differences. Among these factors, only the ascending aorta dimension (36.86±5.21 vs. 39.14 ±5.12, P<0.05), and PGmean (57.96±15.15 vs. 65.11±20.47, P=0.049) differed with statistical significance between both groups. During the TAVI procedure, we recorded the access route and prosthesis size; data showed that most patients in both groups received the transcatheter self-expanded valves (Table 1).
Full table
Procedural outcomes
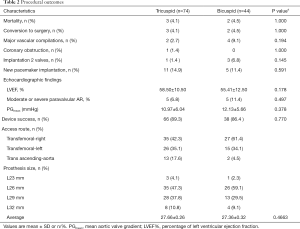
A total of 118 in the intermediate-risk TAVI procedures were performed (Table 2). Five patients were converted to open surgery with complications (3 for cardiac tamponade, 1 for coronary obstruction, 1 for aortic dissection) during the procedure. Unfortunately, those five patients died of treatment for complications. Post-procedure, Bicuspid AS patients compared with tricuspid AS patients had more frequency of Moderate or severe paravalvular AR (6.8% vs. 11.4%), but they differ no significant. The VARC-2 device success rates were 89.3% and 86.4%, respectively. Although the annulus tended to be slightly larger (21.97 vs. 22.70), but more frequent use of the smaller prostheses (27.66 vs. 27.36) in the Bicuspid AS group. There was no significant difference in implantation two valves, major vascular complications, and new peacemaker insertion between the two groups.
Full table
Survival and complications
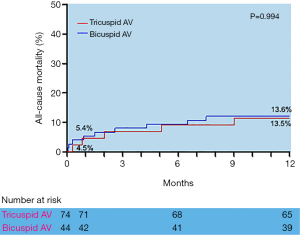
Adverse clinical outcomes at 30 days and 1 year postoperatively in tricuspid AS and Bicuspid AS groups are shown in Table 3. There were no significant differences in 30-day and one-year outcomes postoperatively between the two groups. However, the incidence of major vascular complications at 30-day postoperatively was higher in the Bicuspid AS group, but no difference between the two groups (2.7% vs. 9.1%, P=0.128). Furthermore, the incidence of PPM was scarce (9.5% vs. 6.8% at 30-day, 13.5% vs. 9.1% at 1 year) in the Bicuspid AS group. Finally, the 30-day and 1-year stroke rate (2.7% vs. 2.3% and 5.4% vs. 6.8%) and all-cause mortality (5.5% vs. 4.5% and 13.5% vs. 13.6%) differed without any statistical significance between the two groups.
Full table
During the 1-year follow-up, the log-rank test showed there were no statistically significant differences in the time of death from any cause between the Tricuspid AS and Bicuspid groups (Figure 3), as well as the time of death from cardiovascular or significant complications (Table 3). All the data mentioned above approved the efficacy and safety of supra-annular structure-based sizing strategy in the TAVI procedure.
Functional outcomes
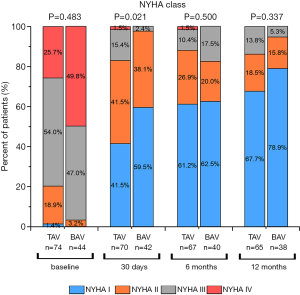
NYHA class was evaluated for the myocardial function of patients on the operation day 30th, 180th, and 360th day. We found the percentage of patients with class III~IV of NYHA dropped after TAVI in both groups, corresponding with better patient states postoperatively. These findings were not statistically significant differences compared to the patients in the TAV group at 1 year (Figure 4).
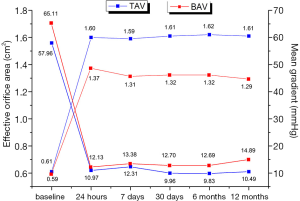
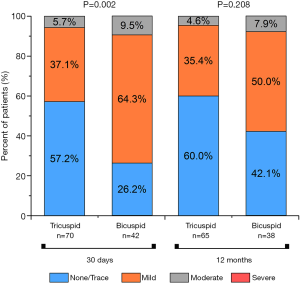
The study described the sequential echocardiographic measurements of PGmean and EOA at the operation day and 1st, 7th, 30th, 180th, and 360th days in both groups to evaluate the bioprosthetic function (Figure 5). Briefly, EOA improved once after the operation in both groups, and constant at 1year, there was no significant difference. Accordingly, PGmean decreased between the two groups at each time, although this change did not differ in 1 year. Compared with the tricuspid AS group patients, Bicuspid group patients had more improvement in PGmean from baseline to 1year (−47.47±13.38 vs. −50.22±19.25 mmHg, P<0.05). Interestingly, in the view of prolonged efficacy, although the result of AR was higher in Bicuspid AS group at 3 months, this improvement and was similar between the two groups at 1 year (Figure 6).
Discussion
In 2010, the first TAVI operation was completed in China. Up to now, more than 4,500 TAVI operations had been conducted in our country, and those concentrated in Beijing, Shanghai, Chengdu, Hangzhou, and other developed areas with high-quality medical resources. Because some prostheses that are popular worldwide (including Medtronic Corvevalve or Edwards Sapien) were not approved by the China food and Drug Administration, the frequently used prosthesis for TAVI in China is the Venus A-valve (through the peripheral vessel route) (18) and J-Valve (through the apex route) (19). However, according to the ACC/AHA guideline, Bicuspid AS with progressive aortic dilatation usually results in the expanded ascending aortic dimension (20), BAV remains to be a relative contraindication for TAVI. Several concerns may explain why BAV patients are precluded from TAVI: (I) heavy calcification of the BAV annulus; (II) elliptical shape size of the BAV annulus; (III) BAV may be associated with other aortic diseases. Statistical data shows that in China, about 50% BAV patients with and without raphe are presented for TAVI, while below 10% BAV patients exclusively with raphe in Western countries (10,21,22). The reasons are complex, possibly due to ethnic and pathogenetic differences, but further study on efficacy and safety of TAVI in Bicuspid AS patients is needed (10). Careful evaluation is vital for achieving satisfactory outcomes when surgery is unsuitable, and TAVI becomes the only choice for the patients. In our study, when the tricuspid AS patients undergoing the same TAVI procedure was compared, the results showed TAVI in Bicuspid AS patients had a similar bioprosthetic function and the incidence of complications after TAVI between the two groups was analogous.
However, Chinese AS patients with BAV are observed to bear the burden of higher aortic valvar calcification, an independent risk factor for essential complications of AR (23,24) and pacemaker implantation (25) as it prevents accurate prosthesis positioning (11). Furthermore, as the aortic valve is anatomically close to the cardiac conduction system, the prosthesis set may cause complete left bundle branch block or atrioventricular block, and the pacemaker implantation were urgent (26). After a statistical analysis of 118 patients in our study, the baseline of Calcium volume was balanced between BAV and TAV groups, and the tendency of pacemaker implantation after TAVI was alike. However, the complication of AR at 30 days postoperatively was more common in the BAV group, which likely resulted from the apparent elliptical shape of the annulus, and the rates of AR at 1 year postoperatively were equal to those observed in long-term safe results. Due to the prolonged hemodynamic stability and favorable clinical prognosis, we could conclude the Venus A-valve was suitable for Chinese AS patients with a higher proportion of BAV and severe calcification.
Certain anatomical criteria should be emphasized for the successful results in BAV patients with predominant AS, since patients with bulky leaflets, enlarged aortic root, dilated ascending aorta, or significant aortic regurgitation are at high risk for procedure failure. Therefore, imaging evaluations of BAV and the associated aortopathy are necessary for the TAVI procedure. CT or magnetic resonance imaging may also be needed to obtain correct anatomical information, apart from routine echocardiography examination. Despite the wide application of TAVI in Bicuspid AS patients, there is still no standard sizing strategy, and operators often empirically select the prosthesis on the MSCT measurements (27). As we know, the information of aortic root cannot precisely reflect the anatomic characteristics of the annulus for prosthesis anchoring (28,29). In this study, to adapt the morphological characteristics of Chinese AS patients with BAV, we utilized the Venus A-Valve through the supra-annular structure-based sizing strategy of TAVI, which was first proposed by Liu et al. (15). They deployed the transcatheter balloon size appropriately to prevent aortic regurgitation to less than mild, which helped select the prosthesis size (prosthesis size determined the valve size according to the instruction of the manufacturer). They also initially demonstrated the feasibility of this advanced strategy in BAV patients with AS with a single-arm, inadequate sample size clinical trial wherein the safety profile was acceptable (15,30). However, the sequential balloon sizing might induce more native valve debris, causing a stroke, which requires a careful operation and observation (31). In our study, as the incidence of stroke in TAV groups was like that observed in published data, the stroke did not occur in the BAV groups, which could eliminate the safety concerns of the supra-annular structure-based sizing strategy.
TAVI was introduced into China in 2010, for the kinds of the self-expandable prosthesis, and another common type of balloon-expandable prosthesis was widely administrated abroad. For the most frequent two devices, PPM and AR, self-expandable prosthesis use was reported to have much lower rates and milder AR than a balloon-expandable prosthesis, especially when compared to the incidence of pacemaker implantation (32). As we know, even moderate aortic regurgitation is independently linked with mid-term mortality, so we chose the former kind (33-35). The Venus A-valve in our study was produced domestically and had completed the TAVI trial in China (ClinicalTrials.gov: NCT01683474) with promising therapeutic efficacy in AS patients. When comparing with the Medtronic CoreValve (Medtronic, Minneapolis, Minnesota, USA), the Venus A-valve yielded favorable clinical outcomes and safety profiles, and the economic advantage was prominent for Chinese patients (18).
There were some limitations in our study. First, some baseline characteristics between the BAV and TAV groups were not parallel, which might have influenced the justification of outcomes. Second, the impact of operators’ experience on sequential balloon sizing and prosthesis choice would be variable. However, we could conquer this problem through extensive practice training. Third, the follow-up period of this study was brief. Therefore, a prospective randomized controlled trial with a prolonged follow-up study to evaluate the efficacy and safety of TAVI in AS patients with BAV is needed.
In summary, our study showed the safety and efficacy of TAVI in nonselective patients with bicuspid AS using a self-expandable valve, which approved the supra-annular structure-based sizing strategy in the procedure and made it feasible for AS patients with BAV in China.
Acknowledgments
Funding: The Tianjin Science and Technology Commission funded this study (grant number 16ZXMJSY00160; 18ZXDBSY00160).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4436
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4436
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4436). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the hospital institutional review board. This study was consistent with the principles of the Helsinki declaration (as revised in 2013) and was approved by the ethics committee of Tianjin Chest Hospital (No: IRB-SOP-016(F)-001-02). Patients voluntarily joined the study, signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clementi M, Notari L, Borghi A, et al. Familial congenital bicuspid aortic valve: a disorder of uncertain inheritance. Am J Med Genet 1996;62:336-8. [Crossref] [PubMed]
- Bob-Manuel T, Heckle MR, Ifedili IA, et al. Outcomes of transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Ann Transl Med 2019;7:102. [Crossref] [PubMed]
- Nistri S, Basso C, Marzari C, et al. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol 2005;96:718-21. [Crossref] [PubMed]
- Patel A, Leon MB. Transcatheter aortic valve replacement in patients with bicuspid aortic valves. J Thorac Dis 2018;10:S3568-72. [Crossref] [PubMed]
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55:2789-800. [Crossref] [PubMed]
- Alegret JM, Ligero C, Vernis JM, et al. Factors related to the need for surgery after the diagnosis of bicuspid aortic valve: one center s experience under a conservative approach. Int J Med Sci 2013;10:176-82. [Crossref] [PubMed]
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Reardon MJ, Adams DH, Kleiman NS, et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2015;66:113-21. [Crossref] [PubMed]
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015;85 Suppl 1:752-61. [Crossref] [PubMed]
- Zhao ZG, Jilaihawi H, Feng Y, et al. Transcatheter aortic valve implantation in bicuspid anatomy. Nat Rev Cardiol 2015;12:123-8. [Crossref] [PubMed]
- Yousef A, Simard T, Webb J, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve: A patient level multi-center analysis. Int J Cardiol 2015;189:282-8. [Crossref] [PubMed]
- Fullerton DA, McIntyre RC Jr. Inhaled nitric oxide: therapeutic applications in cardiothoracic surgery. Ann Thorac Surg 1996;61:1856-64. [Crossref] [PubMed]
- Tchetche D, de Biase C, van Gils L, et al. Bicuspid Aortic Valve Anatomy and Relationship With Devices: The BAVARD Multicenter Registry. Circ Cardiovasc Interv 2019;12:e007107. [Crossref] [PubMed]
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018;91:986-94. [Crossref] [PubMed]
- Patsalis PC, Al-Rashid F, Neumann T, et al. Preparatory balloon aortic valvuloplasty during transcatheter aortic valve implantation for improved valve sizing. JACC Cardiovasc Interv 2013;6:965-71. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438-54. [Crossref] [PubMed]
- Liao YB, Zhao ZG, Wei X, et al. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: Preliminary Experiences in China. Catheter Cardiovasc Interv 2017;89:528-33. [Crossref] [PubMed]
- Luo X, Wang X, Li X, et al. Transapical transcatheter aortic valve implantation using the J-Valve system: A 1-year follow-up study. J Thorac Cardiovasc Surg 2017;154:46-55. [Crossref] [PubMed]
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104-12. [Crossref] [PubMed]
- Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA 2013;310:2069-77. [Crossref] [PubMed]
- Bauer T, Linke A, Sievert H, et al. Comparison of the effectiveness of transcatheter aortic valve implantation in patients with stenotic bicuspid versus tricuspid aortic valves (from the German TAVI Registry). Am J Cardiol 2014;113:518-21. [Crossref] [PubMed]
- Schultz C, Rossi A, van Mieghem N, et al. Aortic annulus dimensions and leaflet calcification from contrast MSCT predict the need for balloon post-dilatation after TAVI with the Medtronic CoreValve prosthesis. EuroIntervention 2011;7:564-72. [Crossref] [PubMed]
- John D, Buellesfeld L, Yuecel S, et al. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc Interv 2010;3:233-43. [Crossref] [PubMed]
- Latsios G, Gerckens U, Buellesfeld L, et al. "Device landing zone" calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter Cardiovasc Interv 2010;76:431-9. [Crossref] [PubMed]
- Bax JJ, Delgado V, Bapat V, et al. Open issues in transcatheter aortic valve implantation. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation. Eur Heart J 2014;35:2639-54. [Crossref] [PubMed]
- Jilaihawi H, Kashif M, Fontana G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275-86. [Crossref] [PubMed]
- Kasel AM, Cassese S, Bleiziffer S, et al. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imaging 2013;6:249-62. [Crossref] [PubMed]
- Piazza N, de Jaegere P, Schultz C, et al. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv 2008;1:74-81. [Crossref] [PubMed]
- Du F, Zhu Q, Jiang J, et al. Incidence and Predictors of Permanent Pacemaker Implantation in Patients Who Underwent Transcatheter Aortic Valve Replacement: Observation of a Chinese Population. Cardiology 2020;145:27-34. [Crossref] [PubMed]
- Auffret V, Regueiro A, Campelo-Parada F, et al. Feasibility, safety, and efficacy of transcatheter aortic valve replacement without balloon predilation: A systematic review and meta-analysis. Catheter Cardiovasc Interv 2017;90:839-50. [Crossref] [PubMed]
- Bapat VN, Attia R, Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: a concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc Interv 2014;7:115-27. [Crossref] [PubMed]
- Abdel-Wahab M, Comberg T, Buttner HJ, et al. Aortic regurgitation after transcatheter aortic valve implantation with balloon- and self-expandable prostheses: a pooled analysis from a 2-center experience. JACC Cardiovasc Interv 2014;7:284-92. [Crossref] [PubMed]
- Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry. Circulation 2014;129:1415-27. [Crossref] [PubMed]
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585-95. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


