Galectins in cancer: carcinogenesis, diagnosis and therapy
Introduction
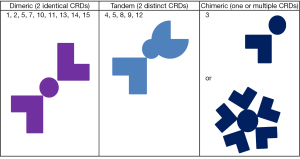
Galectins are a group of proteins known for their ability to bind to B-galactoside sugars, either by N-linked or O-linked glycosylation (1,2). Galectins were first isolated from chick muscle and calf heart and lungs in 1976. Since then, galectins have been named in the order of discovery with as 15 members of the family identified so far. Each galectin has a carbohydrate recognition domain (CRD), and are classified according to the number and structure of these CRD (1,2). Galectins are therefore divided into tandem, dimeric and chimeric galectins (Figure 1). Dimeric galectins have two identical CRD subunits, tandem ones have two distinct CRD subunits and chimeric ones have one or even multiple subunits of the same type (1,2). Galectins-1, -2, -5, -7, -10, -11, -13, -14 and -15 are all dimeric galectins with galectins-4, -5, -8, -9 and -12 falling under the tandem category (1,2). Galectin-3 is the only chimeric galectin discovered in mammals thus far (1,2). In terms of species distribution, galectin-1 (Gal-1), -2, -3, -4, -7, -8, -9, -10, -12 and -13 are present in humans, galectin-5 and -6 in rodents and galectin-11, -14 and -15 were isolated from sheep and goats.
Carcinogenesis, tumor growth and progression are complex and multifactorial processes that depend on an interplay between genotoxic insults, external cellular pressures, tissue micro-environment, and the functionality and modulation of innate cellular repair mechanisms and the body’s immune system. Since their discovery, galectins have been implicated in the development of cancer, the pathogenesis of heart failure and ventricular remodeling, infectious processes, such as HIV, and various other autoimmune and inflammatory processes. Over the past decade the role of galectins in various malignancies has been extensively studied, with the most commonly examined being galectins-3 and -1, followed by -7, -9 and -4. The observation that galectins may have different functions in different tumors adds an additional layer of complexity to their study. For this reason, it is necessary to examine and discuss their potential role in specific neoplasms, separately.
The reproductive system
Galectins in breast cancer
Galectins appear to play an important role in breast cancer progression and metastasis. In a study examining the role of Gal-1 in breast cancer models, investigators found that the frequency of Gal-1 expressing cells in human breast cancer biopsies correlated positively with tumor grade, while specimens from patients with benign hyperplasia showed negative or limited Gal-1 staining (3). Silencing Gal-1 expression in the 4T murine mammary tumor induced a marked reduction in both tumor growth and the number of lung metastasis. Gal-1 attenuation in 4T 1 cells also reduced the frequency of CD4+, CD25+, Foxp3+ T-cells within the tumor draining lymph nodes, spleen and lung metastases (3). Simone and her group studied the function of Gal-3 as a molecular signature of tumors, and showed that Gal-3 B-galactoside binding lectin, coats membranes of most cancer cells and is involved in metastasis, endothelial recognition and evasion of immune surveillance through the killing of activated T cells (4). As early as 2005, Gal-9 was reported to be a good prognostic factor, related to its anti-metastatic potential in breast cancer (5). Gal-7 was initially described as a marker of epithelial differentiation expressed in the stratified epithelium of various tissues (6). In breast cancer Gal-7 expression is significantly augmented in aggressive molecular subtypes, notably in estrogen receptor negative tumors and in cell lines with a basal like phenotype (7). Studies using mouse models have further demonstrated that high expression of Gal-7 was sufficient to increase the metastatic potential of breast cancer cells, rendering them more resistant to apoptosis (7). It has also been shown that p53-induced Gal-7 expression in breast cancer cells correlates with increased NF-κB activity, indicating Gal-7 induction by mutant p53 is dependent on NF-κB activity. In this study NF-κB was found to bind endogenous Gal-7 promoter suggesting Gal-7 could be part of the common pathway used by mutant p53 to promote cancer progression (7). Finally, overexpression of Gal-7 has been shown to enhance spontaneous metastasis of breast cancer cells, especially high grade breast carcinomas, including HER-2 overexpressing and basal like groups (8). In HER-2 overexpressing cases, Gal-7 expression was associated with lymph node axillary metastasis (8).
Galectins in prostate cancer
Gal-1 is the most abundantly expressed galectin in prostate cancer tissue and is markedly upregulated during disease progression (9). In contrast, all other galectins were expressed at lower levels or downregulated during disease evolution, while expression of Gal-8 was unchanged (9). In the same study, Gal-1 expression in human prostate cancer tissue arrays correlated with the presence of angiogenesis, particularly in advanced stages of the disease (9). Silencing Gal-1 expression in prostate cancer cells reduced tumor vascularization without altering expression of other angiogenesis-related genes. In contrast to other cancers, Gal-3 is down regulated in prostate cancer (9). However, Gal-3 molecular cleavage appears to occur during disease progression with higher levels of cleaved Gal-3 correlating with metastatic disease (10). Gal-3 knockdown using small interfering RNA (siRNA) has been associated with reduced cell migration, invasion, cell proliferation, anchorage-independent colony formation, and tumor growth in nude mice (10). Gal-3 knockdown in human prostate cancer PC3 cells has led to cell-cycle arrest at G1 phase, up-regulation of nuclear p21, and hypophosphorylation of the retinoblastoma tumor suppressor protein (pRb), with no effect on cyclin D1, cyclin E, cyclin-dependent kinases (CDK2 and CDK4), and p27 protein expression levels. Raz and coworkers have demonstrated that cleavage of Gal-3 by matrix metalloproteinases (MMP)-2/-9 is associated with angiogenesis, growth and resistance to apoptosis in mouse models of breast and prostate cancers (9,11). Lastly, Knapp et al. have reported the presence of a Gal-3 expression gradient in prostate cancer and benign prostate tissues suggesting Gal-3 expression may be useful in predicting biochemical recurrence (11).
Galectins in cervical cancer
Gal-1 expression has been shown to correlate with the depth of invasion of cervical cancer and the extent of lymph node metastasis (12). Similarly, downregulation of Gal-1 caused a decline in cell growth, proliferation and invasive (12). Huang et al. have shown that Gal-1 mediates radioresistance in cervical cancer cell lines through the H-Ras-dependent pathway involved in DNA damage repair (13). This same group concluded that Gal-1 is an independent prognostic factor in stage I-II cervical cancer patients undergoing definitive radiation therapy (14). In contrast to Gal-1, elevated Gal-7 levels seem to be associated with improved outcomes after definitive radiation therapy in patients with cervical cancer while Gal-9 is inversely associated with cell differentiation and malignant potential of cervical cancer cells (15,16).
Galectins in other cancers of the reproductive system
A correlation between Gal-1 expression and the histopathological grade of vulvar tissues has been suggested, implicating this molecule in the progression of vulvar neoplasia (17).
The respiratory system
Galectins in lung cancer
Various studies have been conducted on the effect of galectins in the pathogenesis, aggression and response to treatment in lung cancer, with Gal-1 being the most investigated. Kuo et al. evaluated the role of Gal-1 in murine lung cancer microenvironment and showed that tumor-derived Gal-1 secretion was associated with tumor-associated dendritic cells (TADCs) production of heparin-binding EGF (HB-EGF)—like growth factor, resulting in tumor progression (18). The association of Gal-1 with integrin α6β4 and Notch1/Jagged2 results in enhanced invasion and migration of lung cancer cells and silencing Gal-1 decreased invasion, migration as well as increase sensitivity to chemotherapy (19,20). Indeed, Gal-1 gene knockdown studies demonstrated increased survival in lung cancer bearing mice (19). A recent study by Carlini et al. examined 103 stage I-III non-small cell lung cancer (NSCLC) patients and found that a higher level of Gal-1 correlated with worse prognosis (21,22). Gal-4 has also been shown to correlate with lymph node metastasis and adverse survival outcomes in lung adenocarcinoma, especially the acinar predominant type (23). Along with histopathological analysis Gal-4 serves as a strong predictor for metastatic potential in this tumor type (23).
The digestive system
Galectins in esophageal and gastric cancers
Gal-7 has been found to be highly expressed in esophageal squamous cell cancers, possibly serving as a biomarker for this disease (24). Gal-7 is also significantly down regulated in gastric cancer cells, suggesting it might play a suppressive role in this neoplasm (25). In contrast, Gal-3 enhances gastric cancer cell motility, possibly by up regulating fascin-1, an actin-binding protein mediating metastatic of the tumor (26).
Galectins in colon cancer
In colon cancer Gal-3 plays an important role in potentiating cell migration and metastasis through activation of certain kinase pathways like the K-Ras-Raf-Erk1/2 pathway (27). Galectins-2, -4, and -8 may also enhance metastasis in colon cancer cells by mediating endothelial cell adhesion (28).
Galectins in pancreatic cancer
With regards to progression of pancreatic cancer, Song et al. has proposed that Gal-3 plays an important role in pancreatic cancer metastasis through activation of Ras, ERK, AKT and Rel A pathways, hence enhancing cell migration and survival (29). Interestingly, Gal-4 may function as a cell-to-cell surface adhesion molecule in pancreatic cancer, preventing pancreatic cell detachment (30).
The nervous system
Galectins in brain tumors
Galectins-1, -3, -4, and -8 have been shown to play a role in the progression of brain gliomas. Gal-1 appears to play a role in augmenting myeloid-derived suppressive cell (MDSC) accumulation and angiogenesis within the glioma microenvironment (31). Silencing Gal-1 expression in their models resulted in prolonged survival, as well as decreased infiltration of the brain with myeloid derived suppressor cells (31). The effect of Gal-1 with regards to response to the chemotherapy agent temozolomide has also been studied. Interestingly, Gal-1 expression was associated with increased transcription of the genes causing temozolomide resistance, thereby suggesting the possibility of interfering with Gal-1 expression as a strategy to modulate temozolomide resistance in brain tumors (32). Gal-3 is abundantly expressed in malignant gliomas, and studies suggest it is found in early preneoplastic lesions. Different from findings in earlier glial lesions, Gal-3 has been shown to be expressed in both neoplastic astrocytes cells and microglia of malignant glioma, suggesting Gal-3 is activated in microglia and macrophages according to disease progression (33).
The skin
Galectins in melanoma
Melanoma is one of the most aggressive cancers, with a very high mortality rate. Gal-3 has been found to play a role in the growth and progression of melanomas. Gal-3 seems to act by modulating the expression of the transcription factor NFAT1 (NFATC2) and regulating autotaxin expression at the transcriptional level (34). Autotaxin stimulates angiogenesis, tumor growth, and metastasis in vivo and silencing Gal-3 results in decreased NFAT1, autotaxin expression and tumor growth (34). Gal-1 expression by tumor and endothelial cells seems to play a role in the resistance of melanoma cells to radiotherapy and chemotherapy through direct modulation of angiogenesis, as well as the immunosuppressive cells in the tumor microenvironment (35,36). Therefore, targeting Gal-1 may represent a potential therapeutic strategy against melanomas (36).
The kidneys
Galectins in renal cell cancers
Gal-3 has been found to be overexpressed in clear cell renal cell carcinoma (CCRCC), compared to normal tissue, with a significantly higher expression in metastatic disease (37,38). Other studies have suggested a protective role for Gal-3 in renal carcinoma cells against apoptosis, as knockdown of Gal-3 expression rendered resistant cells sensitive to arsenic trioxide (39). Gal-3 was found to inhibit ATO-induced apoptosis through enhancing Bcl-2 expression and stabilizing mitochondria (39). This finding implicates Gal-3 in the resistance of renal carcinoma cells to cytotoxic treatment. Gal-1 has been shown to promote tumor progression through upregulation of CXCR4 via NFkB, as evidenced in studies of Gal-1 knockdown in renal carcinoma cells (40). These investigators also found that Gal-1 was overexpressed in renal carcinoma cell lines and in samples from patients with metastasis (40). Knockdown of Gal-1 gene expression in renal cancer cell lines reduced cell invasion, clonogenic ability, and epithelial-mesenchymal transition in vitro, reduced tumor outgrowth in vivo and inhibited the angiogenesis-inducing activity of these cells both in vitro and in vivo (40).
Galectins in bladder cancer
Gal-3 is also highly expressed in both transitional cell and squamous cell carcinomas of the bladder, with higher levels detected in high grade tumors compared to low grade ones (41). Other studies have also confirmed the expression of Gal-1 in bladder cancers and correlated it with tumor grade and metastasis (42).
The endocrine system
Galectins in pituitary tumors
In the case of pituitary tumors Gal-3 seems to be specifically expressed in lactotroph and corticotroph cells, with inhibition of Gal-3 resulting in apoptosis and decreased cell proliferation (43).
Galectins in thyroid and parathyroid cancers
Gal-3 has been shown to be preferentially expressed in malignant thyroid tissue compared to benign thyroid hyperplasia and normal glands (44). A retrospective analysis of Gal-3 and thyroid peroxidase (TP) levels in thyroid cancers demonstrated both Gal-3 and TP are specifically expressed in papillary thyroid carcinoma suggesting these may serve as a diagnostic and prognostic indicator in patients with this histological sub-type (45). Gal-3 overrides the tumor suppressor activity of caveolin-1 (Cav-1) and functions in concert with Cav-1 to promote focal adhesion turnover and tumor cell migration and invasion (46). Thus it appears that Gal-3 and Cav-1 function synergistically to promote focal adhesion signaling, migration and progression of differentiated thyroid cancer (46). Similarly, Gal-3 has been associated with the diagnosis of highly proliferative tumors of the parathyroid gland (47).
Galectins in adrenal tumors
Gal-3, nm23 and COX-2 expression has been reported to be useful in differentiating between malignant and benign pheochromocytomas (48). The combined expression of Gal-3 and COX-2 has been associated with malignant lesions, whereas nm23 expression in the absence of Gal-3 and COX-2 expression indicates a more benign histology (48).
Conclusions: implications of galectin expression and cancer
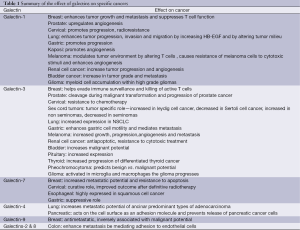
As discussed, galectins serve a fundamental role in the progression of cancer by altering tumor microenvironment, angiogenesis, metastatic potential and modulating immune response (Table 1). The available data indicate a potential role for galectin inhibitors in the treatment of cancer. The cellular effects of Gal-1 and -3 are the most extensively studied and small molecule inhibitors are currently being developed. Importantly, Gal-9 expression has been shown to naturally hinder cancer progression and the development of Gal-9 agonists may offer additional therapeutic opportunities for cancer patients. Additional studies are underway to develop effective pharmacologic inhibitors and agonists of specific galectins and determine their role in cancer care.
Full table
Acknowledgements
Financial support: This work was supported by the Associate Dean for Oncology Programs at TTUHSC, The Billy and Ruby Power Endowment for Cancer Research, The Laura W Bush Institute for Women’s Health, Kiromic, LLC and Endowed Chair for Excellence in Women’s Health Director of Breast Health Service.
Disclosure: The authors declare no conflict of interest.
References
- Cummings RD, Liu FT. Galectins. In: Varki A, Cummings RD, Esko JD, et al. eds. Essentials of Glycobiology. 2nd edition. NY: Cold Spring Harbor Laboratory Press, 2009: Chapter 33.
- Barondes SH, Cooper DN, Gitt MA, et al. Galectins. Structure and function of a large family of animal lectins. J Biol Chem 1994;269:20807-10. [PubMed]
- Dalotto-Moreno T, Croci DO, Cerliani JP, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res 2013;73:1107-17. [PubMed]
- Simone G, Malara N, Trunzo V, et al. Galectin-3 coats the membrane of breast cells and makes a signature of tumours. Mol Biosyst 2014;10:258-65. [PubMed]
- Irie A, Yamauchi A, Kontani K, et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res 2005;11:2962-8. [PubMed]
- Demers M, Rose AA, Grosset AA, et al. Overexpression of galectin-7, a myoepithelial cell marker, enhances spontaneous metastasis of breast cancer cells. Am J Pathol 2010;176:3023-31. [PubMed]
- Campion CG, Labrie M, Lavoie G, et al. Expression of galectin-7 is induced in breast cancer cells by mutant p53. PLoS One 2013;8:e72468. [PubMed]
- Nangia-Makker P, Wang Y, Raz T, et al. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer 2010;127:2530-41. [PubMed]
- Compagno D, Gentilini LD, Jaworski FM, et al. Glycans and galectins in prostate cancer biology, angiogenesis and metastasis. Glycobiology 2014;24:899-906. [PubMed]
- Wang Y, Nangia-Makker P, Tait L, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol 2009;174:1515-23. [PubMed]
- Knapp JS, Lokeshwar SD, Vogel U, et al. Galectin-3 expression in prostate cancer and benign prostate tissues: correlation with biochemical recurrence. World J Urol 2013;31:351-8. [PubMed]
- Kim HJ, Do IG, Jeon HK, et al. Galectin 1 expression is associated with tumor invasion and metastasis in stage IB to IIA cervical cancer. Hum Pathol 2013;44:62-8. [PubMed]
- Huang EY, Chen YF, Chen YM, et al. A novel radioresistant mechanism of galectin-1 mediated by H-Ras-dependent pathways in cervical cancer cells. Cell Death Dis 2012;3:e251. [PubMed]
- Huang EY, Chanchien CC, Lin H, et al. Galectin-1 is an independent prognostic factor for local recurrence and survival after definitive radiation therapy for patients with squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 2013;87:975-82. [PubMed]
- Tsai CJ, Sulman EP, Eifel PJ, et al. Galectin-7 levels predict radiation response in squamous cell carcinoma of the cervix. Gynecol Oncol 2013;131:645-9. [PubMed]
- Liang M, Ueno M, Oomizu S, et al. Galectin-9 expression links to malignant potential of cervical squamous cell carcinoma. J Cancer Res Clin Oncol 2008;134:899-907. [PubMed]
- Kohrenhagen N, Voelker HU, Kapp M, et al. The expression of galectin-1 in vulvar neoplasia. Anticancer Res 2010;30:1547-52. [PubMed]
- Kuo PL, Huang MS, Cheng DE, et al. Lung cancer-derived galectin-1 enhances tumorigenic potentiation of tumor-associated dendritic cells by expressing heparin-binding EGF-like growth factor. J Biol Chem 2012;287:9753-64. [PubMed]
- Hsu YL, Wu CY, Hung JY, et al. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway. Carcinogenesis 2013;34:1370-81. [PubMed]
- Chung LY, Tang SJ, Sun GH, et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res 2012;18:4037-47. [PubMed]
- Kuo P, Bratman S, Shultz D, et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res 2014. [Epub ahead of print]. [PubMed]
- Carlini MJ, Roitman P, Nuñez M, et al. Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung Cancer 2014;84:73-8. [PubMed]
- Hayashi T, Saito T, Fujimura T, et al. Galectin-4, a novel predictor for lymph node metastasis in lung adenocarcinoma. PLoS One 2013;8:e81883. [PubMed]
- Zhu X, Ding M, Yu ML, et al. Identification of galectin-7 as a potential biomarker for esophageal squamous cell carcinoma by proteomic analysis. BMC Cancer 2010;10:290. [PubMed]
- Kim SJ, Hwang JA, Ro JY, et al. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget 2013;4:1461-71. [PubMed]
- Kim SJ, Choi IJ, Cheong TC, et al. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology 2010;138:1035-45.e1-2.
- Wu KL, Huang EY, Jhu EW, et al. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J Gastroenterol 2013;48:350-9. [PubMed]
- Barrow H, Guo X, Wandall HH, et al. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res 2011;17:7035-46. [PubMed]
- Song S, Ji B, Ramachandran V, et al. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS One 2012;7:e42699. [PubMed]
- Belo AI, van der Sar AM, Tefsen B, et al. Galectin-4 Reduces Migration and Metastasis Formation of Pancreatic Cancer Cells. PLoS One 2013;8:e65957. [PubMed]
- Verschuere T, Toelen J, Maes W, et al. Glioma-derived galectin-1 regulates innate and adaptive antitumor immunity. Int J Cancer 2014;134:873-84. [PubMed]
- Le Mercier M, Lefranc F, Mijatovic T, et al. Evidence of galectin-1 involvement in glioma chemoresistance. Toxicol Appl Pharmacol 2008;229:172-83. [PubMed]
- Binh NH, Satoh K, Kobayashi K, et al. Galectin-3 in preneoplastic lesions of glioma. J Neurooncol 2013;111:123-32. [PubMed]
- Braeuer RR, Zigler M, Kamiya T, et al. Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res 2012;72:5757-66. [PubMed]
- Lefranc F, Mathieu V, Kiss R. Galectin-1-mediated biochemical controls of melanoma and glioma aggressive behavior. World J Biol Chem 2011;2:193-201. [PubMed]
- Mathieu V, de Lassalle EM, Toelen J, et al. Galectin-1 in melanoma biology and related neo-angiogenesis processes. J Invest Dermatol 2012;132:2245-54. [PubMed]
- Sakaki M, Fukumori T, Fukawa T, et al. Clinical significance of Galectin-3 in clear cell renal cell carcinoma. J Med Invest 2010;57:152-7. [PubMed]
- Straube T, Elli AF, Greb C, et al. Changes in the expression and subcellular distribution of galectin-3 in clear cell renal cell carcinoma. J Exp Clin Cancer Res 2011;30:89. [PubMed]
- Xu Y, Gu X, Gong M, et al. Galectin-3 inhibition sensitizes human renal cell carcinoma cells to arsenic trioxide treatment. Cancer Biol Ther 2013;14:897-906. [PubMed]
- Huang CS, Tang SJ, Chung LY, et al. Galectin-1 upregulates CXCR4 to promote tumor progression and poor outcome in kidney cancer. J Am Soc Nephrol 2014;25:1486-95. [PubMed]
- Sakaki M, Oka N, Nakanishi R, et al. Serum level of galectin-3 in human bladder cancer. J Med Invest 2008;55:127-32. [PubMed]
- Cindolo L, Benvenuto G, Salvatore P, et al. galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int J Cancer 1999;84:39-43. [PubMed]
- Riss D, Jin L, Qian X, et al. Differential expression of galectin-3 in pituitary tumors. Cancer Res 2003;63:2251-5. [PubMed]
- Chiu CG, Strugnell SS, Griffith OL, et al. Diagnostic utility of galectin-3 in thyroid cancer. Am J Pathol 2010;176:2067-81. [PubMed]
- Weber KB, Shroyer KR, Heinz DE, et al. The use of a combination of galectin-3 and thyroid peroxidase for the diagnosis and prognosis of thyroid cancer. Am J Clin Pathol 2004;122:524-31. [PubMed]
- Shankar J, Wiseman SM, Meng F, et al. Coordinated expression of galectin-3 and caveolin-1 in thyroid cancer. J Pathol 2012;228:56-66. [PubMed]
- Bergero N, De Pompa R, Sacerdote C, et al. Galectin-3 expression in parathyroid carcinoma: immunohistochemical study of 26 cases. Hum Pathol 2005;36:908-14. [PubMed]
- Saffar H, Sanii S, Heshmat R, et al. Expression of galectin-3, nm-23, and cyclooxygenase-2 could potentially discriminate between benign and malignant pheochromocytoma. Am J Clin Pathol 2011;135:454-60. [PubMed]