
Extracellular ubiquitin promotes hepatoma metastasis by mediating M2 macrophage polarization via the activation of the CXCR4/ERK signaling pathway
Introduction
Transfusion is a vital treatment for cancer patients, especially for those with hepatocellular carcinoma (HCC), due to chronic blood loss. However, the safety of transfusion is controversial. Allogeneic blood transfusion can induce transfusion-related immunomodulation (TRIM) responses, including incompatibility issues, allergic reaction, coagulopathy, postoperative infection, multiple organ failure, and cancer recurrence (1-3). These complicated physiologic responses may be induced by apoptotic cells, residual leukocytes, and biological molecules such as soluble mediators, HLA peptides, and cytokines contained in the RBC units (4).
Stored red blood cell (RBC) transfusion has been demonstrated to enhance the risk of recurrence and mortality in multiple cancers (5-9), although its underlying mechanism is still unknown. Ubiquitin (Ub) is a highly conserved 8.5 kDa protein that can be found in all eukaryotes, and its concentration in RBCs is much higher than in other cells (10-12). After 35 days of storage, the hemolysis that occurs in the storage of RBC units has been found to significantly increase the concentration of extracellular ubiquitin (eUb) in whole blood (13,14). The most important function of intracellular Ub is degrading proteins via the ubiquitin-proteasome system (15). The Ub-mediated posttranslational modifications of misfolded and damaged proteins play crucial roles in multiple cellular processes. To date, the effects of intracellular Ub on the regulation of intracellular transformations have been investigated to a relatively considerable degree, but less is known about the functions of extracellular Ub. At our lab, we have demonstrated that eUb can exert immunoregulation effects, such as reinforcing the suppressive effect of Tregs (regulatory T cells), promoting Th cell differentiation into the immunosuppressive Th2 phenotype, and affecting cytokine secretion (14,16).
eUb has been reported to exert pleiotropic functions, including immunoregulatory, anti-inflammatory, and neuroprotective effects, and to regulate the growth and apoptosis of hematopoietic cells and modulate lymphocyte differentiation (17-21). However, studies aimed at investigating the relationship between eUb and the development of cancer are limited. Recently, we have demonstrated that eUb might be the link between allogeneic blood transfusion and poor cancer prognosis; in the melanoma mouse model, eUb promoted tumor metastasis, which might be related to eUb-mediated immunomodulation (22). However, the specific immunoregulatory mechanisms of eUb need to be elucidated in depth. Therefore, this study aimed to confirm the pro-tumor effect of eUb on HCC in vitro and in vivo and to explore the effects of eUb on the regulation of immunocytes.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1054).
Methods
Patient specimens
HCC tissues and the corresponding adjacent nontumor and normal liver tissues were obtained from 40 HCC patients who had undergone surgical resection at the Affiliated Hospital of North Sichuan Medical College. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and the protocol was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (No. 2018-EA-028). Informed consent was obtained from each patient. The clinicopathological features of the HCC patients are shown in Table 1.
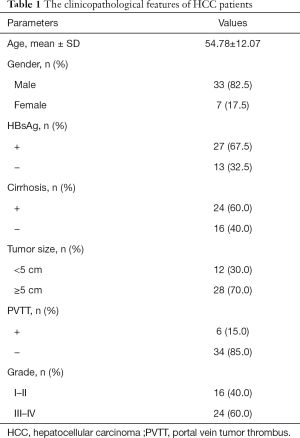
Full table
Cells and reagents
Human hepatoma cell lines HepG2.2.15 and MHCC-97H, mouse hepatoma cell line Hepa1-6, and human monocyte cell line THP-1 were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). HepG2.2.15, MHCC-97H, and Hepa1-6 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (both Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), penicillin (100 U/mL), and streptomycin (100 µg/mL; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The THP-1 cells were maintained in RPMI 1640 medium containing 10% FBS (fetal bovine serum) and penicillin/streptomycin. Cells were incubated in a humidified incubator at 37 °C under 5% CO2. Ub (#U6253), PMA (#P1585), AMD3100 (#A5602), and U0126 (#U120) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All antibodies including mTOR(#2983), p-mTOR (#5536), Akt (#4685), p-Akt (#4060), Vimentin (#5741), E-cadherin (#3195), N-cadherin (#13116), Erk1/2 (#4695), p-Erk1/2 (#4370), Arg-1 (#93668), and GAPDH (#2118) were obtained from Cell Signaling Technology (Beverly, MA, USA). MMP-9 (#76003), iNOS (#178945), TGF-β (#179695), CXCR4 (#181020/124824) and Ub (#7780) were obtained from Abcam (Cambridge, MA, USA).
Animals
Male C57BL/6 mice aged 6 to 8 weeks were purchased from Shanghai Research Center for Southern Model Organisms (Shanghai, China). All animal experiments in this study were approved by the Laboratory Animal Care and Use Committee of Fudan University (No. 2019 Huashan Hospital JS-259) and conducted under the “Guide for the Care and Use of Laboratory Animals” recommended by the US National Institutes of Health.
Tumor metastasis model
Lung metastasis models were established via injection of Hepa 1-6-luciferase cells (2×106) suspended in 300 µL PBS into the lateral tail vein of the mice. The mice were randomly divided into two groups: the eUb group and the control group. Intraperitoneal injections of eUb (U5507; Sigma-Aldrich, St. Louis, MO, USA) and physiological saline were performed 3 times at 24 h intervals. After three weeks of feeding, the tumor metastasis was monitored using the IVIS Spectrum CT (PerkinElmer, Waltham, Massachusetts, USA).
Coculture of hepatoma cells with macrophages
THP-1 cells (1×106) were seeded in Transwell inserts (#3412; Corning, NY, USA) and pretreated with PMA (100 nM) for 24 h to induce M0 macrophages. Next, Ub (10 µg/mL) was used to treat M0 macrophages for 72 h. Meanwhile, HepG2.2.15 and MHCC-97H cells (1×106) were seeded in 6-well plates for 24 h. Then, the Transwell inserts were put into the 6-well plates to enable coculture. After 72 h of coculture, the HepG2.2.15 and MHCC-97H cells were collected for analysis.
Transwell assay
Cells (5×104) suspended in DMEM medium containing 5% FBS were seeded in the upper chamber of Transwell inserts (#3422; Corning, NY, USA). DMEM supplemented with 20% FBS was added to the lower chamber as an attractant solution. After incubation, the remaining cells on the upper chamber were cleared, and the cells that had migrated to the underside of the chamber were fixed with methanol and stained with Giemsa. The migration ability of the cells was measured based on the number of cells that had migrated to the underside of the chamber. Images were captured with an inverted microscope (Olympus, Tokyo, Japan).
Wound-healing assay
Cells (5×105) were seeded in 6-well plates and cultured until a confluent monolayer was formed. A scratch was made with a 10 µL pipette tip. The medium was removed, and the cultures were washed twice with PBS to remove the floating cells. The cells were then cultured in medium containing 5% FBS. The migration ability of the cells was detected by the closure of the scratch. Images were captured with an inverted microscope.
Flow cytometry
Cells (1×106) were suspended in PBS and preincubated with CD86 antibody for 30 min at 4 °C. Next, for intracellular CD206 and CD68 staining, cells were fixed for 15 min with a fixation buffer before pre-cooled 50% methanol was added for 10 min at 4 °C. Then, the cells were washed in PBS with 5% FBS and resuspended with permeabilization buffer before co-staining with the CD206 and CD68 antibodies at RT for 30 min. Finally, the cells were washed and resuspended in PBS, and flow cytometry was performed. The peripheral blood of the mice was collected, and after the supernatants were removed by centrifugation, the ratios of macrophages and Tregs were measured by staining with CD4, CD25, Foxp3+, F4/80, CD11b, CD206, and CD80 antibodies (BioLegend, San Diego, CA, USA), according to the above protocols.
Enzyme-linked immunosorbent assay (ELISA)
The cytokine content was measured using ELISA kits (R&D Systems, Minneapolis, MN, USA). Blood was harvested from the anesthetized mice and centrifuged for 5 min at 3,500 r/min to collect the serum. Meanwhile, the supernatants from the cell culture medium were stored at −80 °C pending assay. ELISA assay was performed according to the manufacturer’s instructions, with each sample detected in triplicate. The concentration of cytokines was calculated using a standard curve.
Immunoprecipitation
Cell lysates were incubated with Ub antibody and normal rabbit IgG overnight at 4 °C and further incubated with agarose (Sigma-Aldrich, St. Louis, MO, USA) for 3 h on a rocking platform. Beads were collected through centrifugation at 12,000 ×g for 1 min at 4 °C, rinsed in wash buffer, and then resuspended in SDS loading buffer. Finally, the lysates were subjected to SDS-PAGE, transferred to PVDF membrane, and detected by immunoblotting with the corresponding antibody.
Hematoxylin and eosin staining
The lung tissues were fixed in 4% formaldehyde and embedded in paraffin. The tissue sections of 5 mm thickness were deparaffinized in xylene, rehydrated in graded alcohol solution, and then stained with hematoxylin and eosin according to the protocol. Images were captured using a light microscope (Olympus, Tokyo, Japan).
Immunofluorescence
The lung tissues were harvested and incubated with CD206 antibody overnight at 4 °C. After, the tissues were washed and incubated 1 h with FITC goat anti-mouse IgG secondary antibody at 37 °C in the dark. Nuclei were stained with DAPI for 10 min. Images were captured using an inverted fluorescence microscope (Olympus, Tokyo, Japan).
qPCR
Total RNA was extracted with TRIzol (Invitrogen, California, USA) and reverse transcribed to cDNA by PrimeScript™ Reverse Transcriptase (Takara Bio, Shiga, Japan). qPCR was performed with SYBR® Premix Ex Taq™ II (Takara Bio, Shiga, Japan) and the LightCycler® 480 System (Roche, Basel, Switzerland). The method of 2−∆∆Ct was used to analyze the mRNA level. The relative expressions of all genes were quantified as fold changes versus control. GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) was used as internal control. Table 2 shows the primers used for qPCR.
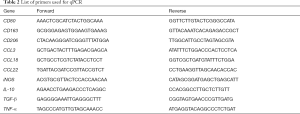
Full table
Western blot
Cells were washed with cold PBS, and the extracts were prepared using lysis buffer. The lung tissues from the mice were also prepared. The protein extracts were subjected to SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% BSA for 1 h at RT and then incubated overnight using primary antibody at 4 °C. After that, the membranes were washed and incubated with the second antibody conjugated to horseradish peroxidase at RT for 1 h. The protein signal was checked with Fusion FX7 (VILBER, Paris, France).
Statistical analysis
GraphPad Prism 7 (GraphPad Software, La Jolla, CA) was used to perform statistical analysis. Non-parametric data (the expressions of CXCR4 and Ub in clinical tissues) were shown as median (25th to 75th interquartile range), and comparisons between groups were performed with Wilcoxon matched-pairs signed rank test and Kruskal-Wallis test. Correlation of non-parametric data were analyzed using Spearman Correlation test. Parametric data were expressed as mean ± standard deviation (SD) and comparisons between groups were performed with Student’s t-test and one-way ANOVA. A P value of <0.05 was considered statistically significant.
Results
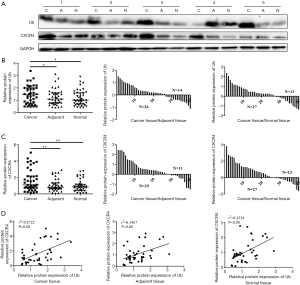
Ub was upregulated in HCC tissues and positively related with CXCR4
Since eUb was confirmed to promote tumor metastasis in a melanoma mouse model in our previous study, we wanted to explore further the role of Ub in the development and progression of HCC. First, we examined Ub expression in human HCC tissues and the corresponding adjacent nontumor and normal liver tissues by Western blot. The results showed that the expressions of Ub and CXCR4 were strikingly stronger in HCC tissues than in adjacent nontumor and normal liver tissues (Figure 1A), and there was no significant difference between their expressions in adjacent nontumor tissues and normal liver tissues (Figure 1B,C). Moreover, there was a positive correlation between Ub and CXCR4, and their correlation in HCC tissues was stronger than in adjacent nontumor and normal liver tissues (Figure 1D). Together, these results indicated that Ub might participate in the development and progression of HCC.
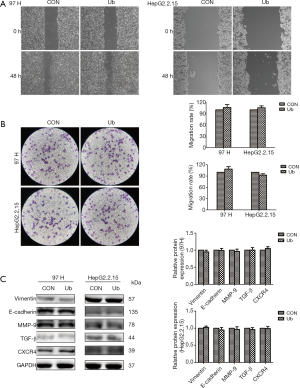
eUb had no significant effect on the migration of hepatoma cells
To determine whether eUb participates in the development and progression of HCC, the effect of eUb on the migration ability of hepatoma cells (MHCC-97H and HepG2.2.15) was observed in vitro. After treatment with eUb (10 µg/mL) for 48 h, the Transwell assay showed that eUb did not significantly affect the mobility of 97H and HepG2.2.15 (Figure 2A). The same result was obtained from the wound-healing assay (Figure 2B). We also detected the expressions of Vimentin and E-cadherin, which are the key proteins in epithelial-mesenchymal transition, as well as those of MMP-9 and TGF-β, which are related to tumor metastasis. The data showed no obvious changes in the expressions of the proteins. Meanwhile, CXCR4, which is involved in cancer metastasis, was not significantly changed by eUb exposure (Figure 2C). Collectively, the above results demonstrated that eUb had no direct effect on the migration of hepatoma cells in vitro.
eUb enhanced the migration of hepatoma cells via the involvement of macrophages
Tumor-related macrophages have been shown to participate in tumor development and progression, and eUb possibly regulates the differentiation and function of immunocytes. Therefore, we investigated whether eUb could affect the migration of hepatoma cells via the involvement of macrophages. Since the differentiation of THP-1 monocytes has been used as a macrophage model in vitro, THP-1 cells were differentiated into macrophages through incubation with PMA. After 24 h, the THP-1 cells were adherent and were designated as M0 phenotype macrophages. The M0 macrophages were treated with eUb (10 µg/mL) for 72 h, and then cocultured with 97H and HepG2.2.15 cells via Transwell inserts. The migration abilities of the 97H and HepG2.2.15 cells were measured after 72 h coculture. The results showed that the mobility of hepatoma cells was increased after coculture with macrophages pretreated with eUb compared to that of the hepatoma cells cocultured with the control macrophages treated with physiological saline (Figure 3A,B). Besides, the expressions of Vimentin, E-cadherin, MMP-9, TGF-β, and CXCR4 were elevated simultaneously (Figure 3C). These findings indicated that eUb could enhance the migration of hepatoma cells via the involvement of macrophages.
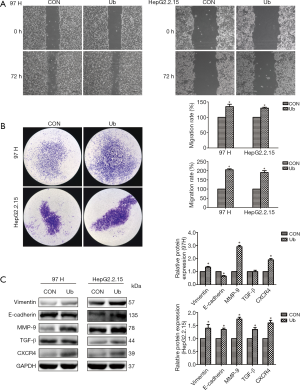
eUb promoted the metastasis of hepatoma cells in the tumor-bearing model
A murine lung metastasis model was established to investigate whether eUb promoted the metastasis of hepatoma cells in vivo. After three weeks of feeding, the bioluminescent signal was much stronger in the lungs of the eUb-treated mice than in those of the control mice (Figure 4A). Furthermore, a greater number of metastatic nodules were found in the lungs of the Ub-treated mice than in those of the control mice through H&E staining (Figure 4B). Meanwhile, we harvested the lung tissues to detect the expressions of metastasis-related proteins and found that E-cadherin was downregulated; however, Vimentin, N-cadherin, and CXCR4 were upregulated in the eUb group compared to the control group. Moreover, the expressions of p-mTOR and p-Akt, which are related to tumor proliferation, were significantly upregulated in the eUb-treated mice (Figure 4C). These results, taken together, suggested that eUb promoted the metastasis of hepatoma cells in the tumor-bearing model.
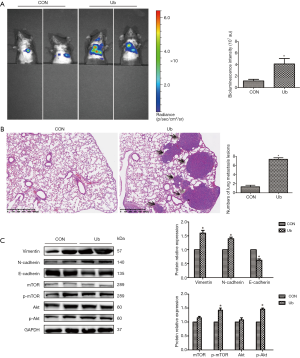
eUb upregulated the ratio of M2 macrophages in tumor-bearing mice
To determine whether the eUb-induced metastasis of hepatoma cells in vivo was correlated with the M2 macrophage polarization, we collected the peripheral blood of the tumor-bearing mice and analyzed the ratio and polarization of macrophages by flow cytometry. The data showed that the percentages of M0 and M1 macrophages were not obviously changed, while the ratio of M2 macrophages was significantly elevated in the eUb-treated mice (Figure 5A). We also measured the percentages of CD4+ lymphocytes and Tregs, which were not significantly changed after eUb intervention (Figure 5B). Meanwhile, the immunofluorescence assay demonstrated that the proportion of M2 macrophages in lung tissues was increased in the eUb-treated group (Figure 5C). The concentration of TGF-β in the serum of the eUb-treated mice was upregulated; however, the concentrations of TNF-α and IL-10 were not obviously changed compared with the control mice (Figure 5D). Furthermore, the results of Western blot showed that iNOS (M1 macrophage marker) and Arg-1 (M2 macrophage marker) were both enhanced upon eUb exposure; yet, Arg-1 was more significantly upregulated than iNOS (Figure 5E). These data taken together indicated that eUb upregulated the ratio of M2 macrophages in tumor-bearing mice.
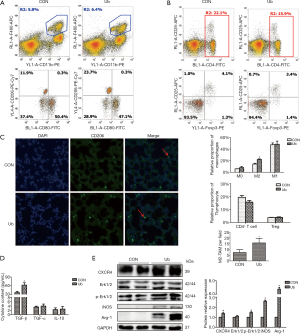
eUb promoted M2 macrophage polarization
Since coculture with eUb-treated macrophages promoted the migration of hepatoma cells, and macrophage polarization could affect the development and progression of cancer, we specifically explored the effect of eUb on macrophage polarization. The M0 macrophages differentiated from THP-1 cells were treated with different concentrations of eUb (1, 2, 5, and 10 µg/mL) for 72 h, and we assessed the polarization of the macrophages by measuring the mRNA levels of several classical macrophage markers using qPCR. The expressions of M1 markers (TNF-α, iNOS, CD80, and CCL3) were significantly downregulated by eUb; however, the expressions of M2 markers (CD163, CD206, CCL22, IL-10, TGF-β, and CCL18) were increased (Figure 6A). The expressions of CD86 (M1 macrophage marker) and CD206 (M2 macrophage marker) were also measured by flow cytometry. CD206 expression was obviously increased while there was no change in CD86 expression after eUb exposure (Figure 6B). From these results, we concluded that eUb promoted macrophages polarization towards the M2 phenotype.
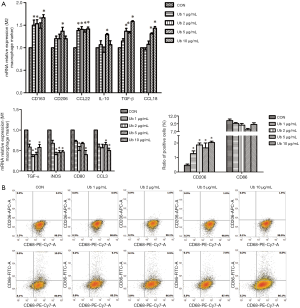
eUb might promote M2 macrophage polarization via the CXCR4/ERK pathway
In the subsequent experiments, we explored the specific mechanisms by which eUb promoted M2 macrophage polarization. We confirmed the binding of Ub and CXCR4 (Figure 7A) and observed the upregulation of both CXCR4 and p-Erk1/2 in macrophages after treatment with eUb (Figure 7B). Meanwhile, the expressions of CXCR4 and p-Erk1/2 were increased in the lung tissues of eUb-treated mice compared with the control mice (Figure 5E). Therefore, we utilized AMD3100 (CXCR4 inhibitor) and U0126 (Erk1/2 inhibitor) respectively, to explore whether the CXCR4/Erk pathway was involved in eUb-stimulated M2 macrophage polarization.
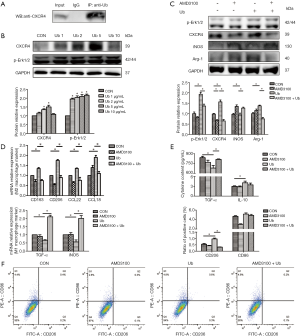
The M0 macrophages were incubated with eUb (10 µg/mL) for 72 h after pretreated by AMD3100 (100 µM) and U0126 (10 µM) for 30 min. The results of Western blot showed that AMD3100 inhibited the eUb-induced increase in the expressions of CXCR4, p-Erk1/2, and Arg-1, in contrast to the alteration of iNOS (Figure 7C). The results of qPCR also showed that the AMD3100 preincubation attenuated the upregulation of M2 macrophage markers (CD163, CD206, CCL22, and CCL18), and stimulated the downregulation of M1 macrophage markers (TNF-α and iNOS) induced by eUb (Figure 7D). Moreover, ELISA analysis showed that the AMD3100 pretreatment upregulated the decrease in TNF-α induced by eUb, but it had no influence on the concentration of IL-10 (Figure 7E). Finally, AMD3100 inhibited the eUb-mediated increase in CD206, while CD86 was not dramatically affected by eUb and AMD3100 exposure (Figure 7F).
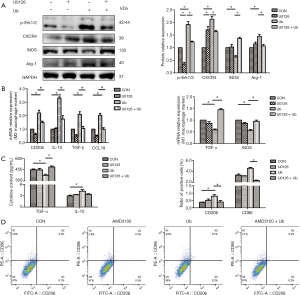
Besides, in Western blot analysis, the preincubation of Erk1/2 inhibitor U0126 decreased the upregulation of p-Erk1/2, CXCR4, and Arg-1 and enhanced the downregulation of iNOS induced by eUb (Figure 8A). The qPCR results showed that U0126 attenuated the upregulation of M2 macrophage markers (CD206, IL-10, TGF-β, and CCL18) and stimulated the downregulation of M1 macrophage markers (TNF-α and iNOS) induced by eUb (Figure 8B). Moreover, U0126 reversed the eUb-mediated alterations of TNF-α and CD206 but it did not affect the expressions of IL-10 and CD86 in ELISA and flow cytometry analysis, respectively (Figure 8C,D). Collectively, the above findings indicated that the CXCR4/Erk pathway was involved in the polarization of macrophage and that by inhibiting the CXCR4/Erk pathway, M2 macrophage polarization stimulated by eUb could be attenuated.
Discussion
Because of the upregulation of Ub in body fluids seen in many diseases, eUb is identified as a disease biomarker (23). We found that Ub was elevated in HCC tissues compared with the adjacent nontumor and normal liver tissues, Wang et al. also confirmed that Ub was upregulated in HCC specimens and that the expression of Ub was negatively associated with the clinicopathological degree of HCC, as well as breast cancer, rectal cancer, prostate cancer, and ovarian cancer (24). Meanwhile, CXCR4 was upregulated in HCC tissues, and there was a positive correlation between Ub and CXCR4. CXCR4 is a 7-transmembrane G protein-coupled receptor that is expressed on numerous cells, including cancer cells, lymphocytes, and monocytes (25). CXCR4 was reported to correlate with the proliferation and invasiveness of cancer cells. Yang et al. confirmed the increased expression of CXCR4 in high-grade chondrosarcoma tissues compared with low-grade specimens and that its expression was closely related with cancer recurrence (26); Jiang et al. also indicated that the high CXCR4 expression in gastric, colorectal, and esophageal cancer could predict a poor prognosis (27). eUb is considered to be an endogenous agonist of CXCR4, and the binding of eUb and CXCR4 initiates Ca2+ influx into the cells and induces chemotactic response (28). Moreover, Ub-CXCR4 interaction was suggested to contribute to lung tumor metastasis induced by acute lung infection (29). Taken together, the pro-tumor effect of eUb could be related to the involvement of CXCR4.
Sudzhashvili et al. demonstrated that eUb injection stimulated the proliferation of hepatectomized alcoholic liver cells (30), while Scofield et al. found that eUb alone had no effect on the proliferation of fibroblasts (31). eUb has also been shown to inhibit the cardiac fibrosis stimulated by β-AR (32), and to inhibit the growth of KT-3 and HL-60 cells through the degradation of STAT3 (22). All of these studies suggested that the cell-type-specific roles of eUb are affected by the fundamental pathological state (33). In this study, we found that eUb had no effect on the migration of hepatoma cells directly in vitro; meanwhile, the metastasis ability of hepatoma cells was enhanced after coculture with macrophages pretreated with eUb, which indicated that eUb might regulate the function of macrophages to affect hepatoma metastasis.
Tumor development and progression depend on the local tumor microenvironment, which includes lymphocytes, macrophages, monocytes, myofibroblasts, fibroblasts, mesenchymal stem cells, and the extracellular matrix. The macrophages within the tumor microenvironment are termed as tumor-associated macrophages (TAMs), which are crucial regulators of the intricate interaction between cancer and the immune system (34). TAMs are usually divided into two phenotypes: (I) M1 macrophages, which can be induced by LPS alone or in combination with Th1 cytokines such as GM-CSF and IFN-γ and secrete pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-12; and (II) M2 macrophages, which can be induced by Th2 cytokines such as IL-13 and IL-4 and release anti-inflammatory cytokines, including TGF-β and IL-10. Macrophages can switch from one phenotype to another in a process called “macrophage polarization” (35). Multiple studies have indicated that TAMs in malignant cancers commonly display M2-like phenotypes, which contribute to an immunosuppressive microenvironment and promote tumor development and progression (36). In our study, we not only demonstrated eUb could promote the metastasis of hepatoma cells in vivo but also confirmed the upregulation of M2 macrophages in lung tissues and peripheral blood of tumor-bearing mice after eUb intervention. Furthermore, we found the macrophages displayed M2 phenotype after eUb treatment in vitro. Therefore, we concluded that eUb might promote M2 macrophage polarization to fuel the metastasis of HCC.
The potential underlying mechanism of M2 macrophage polarization mediated by eUb was also explored in vitro. We found CXCR4 and p-Erk1/2 to be elevated after eUb exposure, and the utilization of CXCR4 and Erk1/2 inhibitors blunted the M2 macrophage polarization induced by eUb. Meng et al. reported that the increased p-Erk1/2 expression in the tumor vasculature was related to the higher expression of CXCR4 (37). Extracellular signal-regulated protein kinases 1 and 2 (Erk1/2) are serine/threonine kinases involved in cell growth and differentiation (38). Richardson et al. demonstrated that Erk1/2 exerts a crucial role in driving M-CSF-dependent proliferation of bone marrow-derived macrophages (38). Meanwhile, in breast cancer, the tumor-derived lactate could induce M2 macrophage polarization via the activation of the Erk/STAT3 pathway (34). More importantly, Zhang et al. reported that the MAPKs (mitogen-activated protein kinases) pathway was involved in hypoxia-induced M2 macrophage polarization, and although three MAPKs, JNK, Erk1/2, and p38 were activated under hypoxia, only the Erk1/2 inhibitor repressed the M2 macrophage polarization induced by hypoxia (39). Collectively, we hold the opinion that eUb could activate the CXCR4/ERK pathway to mediate M2 macrophage polarization and promote hepatoma metastasis.
Conclusions
Our study not only demonstrated that eUb could contribute to the hepatoma metastasis via the involvement of M2 macrophage polarization, but also identified that the activation of CXCR4/ERK signaling pathway could be the underlying mechanism of eUb-mediated M2 macrophage polarization. These findings may provide laboratory evidence of the link between cancer recurrence and TRIM induced by stored RBCs, indicating that personalized transfusion strategies are needed for the treatment of HCC patients. Neutralizing Ub in stored RBC units could lessen the detrimental clinical outcomes induced by stored RBC transfusion.
Acknowledgments
Funding: This study was supported by the Natural Science Foundation of China (Grant No. 81670173 and 81570166) and Public Health Leading Academic Discipline Project supported by Shanghai Municipal Commission of Health and Family Planning (Grant No. 15GWZK0501).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1054
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1054
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-1054
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1054). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal protocols were approved by the Laboratory Animal Care and Use Committee of Fudan University (No. 2019 Huashan Hospital JS-259) and conducted under the “Guide for the Care and Use of Laboratory Animals” recommended by the US National Institutes of Health. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and the protocol was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (No. 2018-EA-028). Informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lelubre C, Vincent JL. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Crit Care 2013;17:R66. [Crossref] [PubMed]
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008;358:1229-39. [Crossref] [PubMed]
- van Straten AH, Soliman Hamad MA, van Zundert AA, et al. Effect of duration of red blood cell storage on early and late mortality after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2011;141:231-7. [Crossref] [PubMed]
- Goubran H, Sheridan D, Radosevic J, et al. Transfusion-related immunomodulation and cancer. Transfus Apher Sci 2017;56:336-40. [Crossref] [PubMed]
- Cata JP, Wang H, Gottumukkala V, et al. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth 2013;110:690-701. [Crossref] [PubMed]
- Abu-Ghanem Y, Zilberman DE, Dotan Z, et al. Perioperative blood transfusion adversely affects prognosis after nephrectomy for renal cell carcinoma. Urol Oncol 2018;36:12.e15-12.e20. [Crossref] [PubMed]
- Kneuertz PJ, Patel SH, Chu CK, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol 2011;18:1327-34. [Crossref] [PubMed]
- Wang CC, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2009;16:1832-42. [Crossref] [PubMed]
- Squires MH 3rd, Kooby DA, Poultsides GA, et al. Effect of Perioperative Transfusion on Recurrence and Survival after Gastric Cancer Resection: a 7-Institution Analysis of 765 Patients from the US Gastric Cancer Collaborative. J Am Coll Surg 2015;221:767-77. [Crossref] [PubMed]
- Haas AL. Ubiquitin-mediated processes in erythroid cell maturation. Adv Exp Med Biol 1991;307:191-205. [Crossref] [PubMed]
- Takada K, Nasu H, Hibi N, et al. Serum concentrations of free ubiquitin and multiubiquitin chains. Clin Chem 1997;43:1188-95. [Crossref] [PubMed]
- Haas AL, Wilkinson KD. The large scale purification of ubiquitin from human erythrocytes. Prep Biochem 1985;15:49-60. [Crossref] [PubMed]
- Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res 2006;135:226-32. [Crossref] [PubMed]
- Zhu X, Yu B, You P, et al. Ubiquitin released in the plasma of whole blood during storage promotes mRNA expression of Th2 cytokines and Th2-inducing transcription factors. Transfus Apher Sci 2012;47:305-11. [Crossref] [PubMed]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature 2003;426:895-9. [Crossref] [PubMed]
- Cao Y, Li C, Zhang Q, et al. Extracellular ubiquitin enhances the suppressive effects of regulatory T cells on effector T cell responses. Clin Lab 2014;60:1983-91. [Crossref] [PubMed]
- Majetschak M, Krehmeier U, Bardenheuer M, et al. Extracellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood 2003;101:1882-90. [Crossref] [PubMed]
- Majetschak M, Cohn SM, Nelson JA, et al. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery 2004;135:536-43. [Crossref] [PubMed]
- Pancré V, Pierce RJ, Fournier F, et al. Effect of ubiquitin on platelet functions: possible identity with platelet activity suppressive lymphokine (PASL). Eur J Immunol 1991;21:2735-41. [Crossref] [PubMed]
- Scofield SL, Amin P, Singh M, et al. Extracellular Ubiquitin: Role in Myocyte Apoptosis and Myocardial Remodeling. Compr Physiol 2015;6:527-60. [Crossref] [PubMed]
- Daino H, Matsumura I, Takada K, et al. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood 2000;95:2577-85. [Crossref] [PubMed]
- Zhang J, Chen S, Yan Y, et al. Extracellular Ubiquitin is the Causal Link between Stored Blood Transfusion Therapy and Tumor Progression in a Melanoma Mouse Model. J Cancer 2019;10:2822-35. [Crossref] [PubMed]
- Sujashvili R. Advantages of Extracellular Ubiquitin in Modulation of Immune Responses. Mediators Inflamm 2016;2016:4190390.
- Wang J, Liu Y, Jiang X, et al. Expression of ubiquitin and P27 protein in hepatocellular carcinoma and its clinicopathlogic significance Journal of Shanxi Medical University 2010;41:116-9. (in Chinese).
- Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem 2010;285:15566-76. [Crossref] [PubMed]
- Yang P, Wang G, Huo H, et al. SDF-1/CXCR4 signaling up-regulates survivin to regulate human sacral chondrosarcoma cell cycle and epithelial-mesenchymal transition via ERK and PI3K/AKT pathway. Med Oncol 2015;32:377. [Crossref] [PubMed]
- Jiang Q, Sun Y, Liu X. CXCR4 as a prognostic biomarker in gastrointestinal cancer: a meta-analysis. Biomarkers 2019;24:510-6. [Crossref] [PubMed]
- Saini V, Staren DM, Ziarek JJ, et al. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1α function through distinct receptor interactions. J Biol Chem 2011;286:33466-77. [Crossref] [PubMed]
- Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res 2013;19:4706-16. [Crossref] [PubMed]
- Sudzhashvili RSh, Bakuradze ED, Modebadze IR, Dekanoidze DM. Ubiquitin in combination with alcohol stimulates proliferative activity of hepatocytes. Georgian Med News 2013.86-90. [PubMed]
- Scofield SLC, Daniels CR, Dalal S, et al. Extracellular ubiquitin modulates cardiac fibroblast phenotype and function via its interaction with CXCR4. Life Sci 2018;211:8-16. [Crossref] [PubMed]
- Daniels CR, Foster CR, Yakoob S, et al. Exogenous ubiquitin modulates chronic β-adrenergic receptor-stimulated myocardial remodeling: role in Akt activity and matrix metalloproteinase expression. Am J Physiol Heart Circ Physiol 2012;303:H1459-68. [Crossref] [PubMed]
- Steagall RJ, Daniels CR, Dalal S, et al. Extracellular ubiquitin increases expression of angiogenic molecules and stimulates angiogenesis in cardiac microvascular endothelial cells. Microcirculation 2014;21:324-32. [Crossref] [PubMed]
- Mu X, Shi W, Xu Y, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018;17:428-38. [Crossref] [PubMed]
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018;233:6425-40. [Crossref] [PubMed]
- Liu H, Dong H, Jiang L, et al. Bleomycin inhibits proliferation and induces apoptosis in TPC-1 cells through reversing M2-macrophages polarization. Oncol Lett 2018;16:3858-66. [PubMed]
- Meng YM, Liang J, Wu C, et al. Monocytes/Macrophages promote vascular CXCR4 expression via the ERK pathway in hepatocellular carcinoma. Oncoimmunology 2017;7:e1408745. [Crossref] [PubMed]
- Richardson ET, Shukla S, Nagy N, et al. ERK Signaling Is Essential for Macrophage Development. PLoS One 2015;10:e0140064. [Crossref] [PubMed]
- Zhang J, Cao J, Ma S, et al. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget 2014;5:9664-77. [Crossref] [PubMed]


