
Innate immune checkpoints for cancer immunotherapy: expanding the scope of non T cell targets
With the recent progresses in the field of immuno-oncology, harnessing the power of the immune system to fight cancer has become one of the pillars of cancer care, together with the traditional approaches of surgery, chemotherapy, targeted oncogene pathway inhibition, and radiation therapy. Treatment with monoclonal antibodies against immune checkpoint targets on T-cells, including the programmed cell death protein 1 (PD-1) pathway, was proven clinically effective in a variety of cancers and was approved for several indications over the past few years (1). However, only a subset of patients respond to immunotherapy and complete response remains uncommon across cancer types (2). Among the mechanisms of resistance to immunotherapy, the establishment of a highly immunosuppressive tumor microenvironment (TME), composed of cells such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), cancer-associated fibroblasts and M2 macrophages (TAMs), was reported as having a major role in limiting effective antitumor immunity (3,4). Tumor-associated myeloid cells, particularly TAMs, constitute a major component of the TME and recent studies support their key contribution to the suppression of CD8+ T-cell function and is associated with poor prognosis in many cancers (5,6). Taking advantage of the highly plastic nature of myeloid cells, a number of strategies to reprogram the function of innate immunity towards an immunostimulatory state have been attempted to enhance the activity of checkpoint inhibitors in cancer. Those include targeting PI3Kγ (7,8), CSF-1R (9,10), IDO (11), VEGF/VEGFR (12,13) or CD40 (14-16).
Many pathways expressed by TAMs have been shown to control their phenotypic state, either by directly inhibiting effector cell activity (phagocytosis, antigen-presentation, cytokine production) or by promoting cell expansion, infiltration and activity (17). Proteins expressed on the surface of tumor cells, such as CD47, PD-L1 and VISTA, have the ability to trigger inhibitory “don’t eat me” signals on TAMs and protect tumors from immunosurveillance mechanisms. Approaches pioneered by Dr. Weissman’s research group at Stanford University found that disruption of the interaction between CD47 and signal-regulatory protein α (SIRPα), expressed on CD11b+ myeloid cells [including macrophages and dendritic cells (DCs)], effectively enhances phagocytosis toward tumor cells in vitro and reduces tumor growth in vivo (18-24). However, variations of the responsiveness to CD47-SIRPα blockade exist (21,25). In this new manuscript by Barkal et al., the authors describe CD24 as a novel don’t eat me signal expressed in several cancers, particularly ovarian (OC) and triple-negative breast cancers (TNBC) (25). CD24 is a heavily glycosylated glycosylphosphatidylinositol-anchored surface protein that was shown previously to interact with the inhibitory receptor sialic-acid-binding Ig-like lectin 10 (Siglec-10), which is expressed on the surface of TAMs (26,27). Through binding to Siglec-10, CD24 elicits an inhibitory signal by activating the phosphatases-SHP-1 and/or SHP-2. The interaction between CD24 and Siglec-10 was first identified and described to negatively regulate the Danger-associated molecular patterns (DAMPs) signaling through toll-like receptors (TLRs) in dendritic cells (DCs) (26).
Although authors in this paper mainly focused on the characterization of CD24 as a don’t-eat-me signal, the functional contribution of CD24 as a sensor for the DAMPs signals certainly deserves further investigation since DAMP signals are found to be released from necrotic tumor cells upon anti-cancer therapies (28-30). Proteins that similarly act as negative regulators of TLR-signaling, such as IL-37 (31) and SIGIRR/IL-1R8 (32,33), have already been described to act in the cancer-immune crosstalk with potential implications in therapeutic efficacy. In fact, the release of certain DAMP molecules such as high mobility group box 1 (HMGB1) is a key component of an immunogenic type of cell death triggered by anticancer agents—such as some classes of chemotherapy (anthracyclines, oxaliplatin and bortezomib) and radiotherapy—which mediate their efficacy by enhancing antitumor immunity (34,35).
Barkal et al. describe CD24 initially as a highly expressed transcript across several cancer types, particularly TNBC and OC, and an association between its expression and poorer prognosis. Interestingly, CD24 expression in TNBC cells appeared substantially higher than classic immune checkpoints on tumor cells such as PD-L1 and with higher specificity than CD47, in the TNBC patient population tested. However, as opposed to PD-L1—whose expression presents an inducible nature in response to inflammatory stimulus such as IFNγ—no discussion regarding the potentially inducible nature of CD24 was presented. In addition, the remaining evidence presented of high CD24 expression in ovarian and breast tumor cells and Singlec-10 expression in TAMs by FCS raise questions as no comparisons with other tumor cell or immune cell populations are made. Similarly, even though the expression of Siglec-10 in macrophages is shown to be dependent on M2 macrophage-polarizing cytokines (such as IL-10, TGF-b and IL-4), the exact mechanism by which this inhibitory pathway is engaged is still unknown.
Considering the fact that Siglec-10—like other members of the Siglecs family (sialic-acid-binding immunoglobulin-like lectins) which exhibit preferential binding to sialylated proteins—binds to sialylated CD24 with higher affinity and that the sialylation of CD24 contributes to the suppression of tumor cell phagocytosis by macrophages (25), the authors highlight an important—yet not much explored—mechanism of tumor cell hyper-sialylation in the suppression of innate immunity. While heavy glycosylation is known as a tumor cell feature, aberrant sialylation is appreciated as the most consistent and prominent form of glycosylation among different tumor types (36). Therefore, if sialylation of proteins expressed on tumor cells suppress phagocytosis to a certain degree, the implication of the following should be considered. (I) Inhibition of sialyltransferase expression: Since there has been at least 9 sialyltransferases characterized to be essential in catalyzing the linkage of sialic acids onto the growing glycan structures during malignant tumor progression (36-38), blocking these sialyltransferases is likely to help reducing the sialylation and hence improving the tumor cell phagocytosis by macrophages. (II) Blockade of hexosamine biosynthesis pathway: it has been shown that tumor cells can utilize the hexosamine metabolism pathway driven by certain oncogenic stimuli to increase the production of cytosine monophosphate (CMP)-sialic acid and therefore sialylated glycoconjugates (36,39,40), the blockade of this pathway is hence worthwhile considering to reducing the surface sialylation on tumor cells.
Further in vitro studies testing the therapeutic potential of CD24 blockade with monoclonal antibodies in order to disrupt CD24-Singlec-10 signaling demonstrated enhanced tumor cell engulfing by TAM, using for instance models of labeled human TNBC cells (MCF-7). Not surprisingly, induction of phagocytosis by anti-CD24 treatment was apparently dependent on the expression of CD24 on tumor cells and was largely increased upon addition of CD47 blocking antibodies. Interestingly, the synergy observed by the authors between anti-CD24 and anti-cancer agents (cetuximab) suggest again that studies evaluating the modulatory role of CD24 over DAMP signaling might be a promising avenue of research. In their final results, the authors explore the inhibitory role of CD24 on phagocytosis using in vivo models. The findings go along with their previous results and reinforce the assumption that the macrophage-dependent clearance of tumor cells relies on CD24 expression. It is important to note that no experiment was performed to address in a more definitive way the contribution of CD24-Singlec-10 interactions in the outcome of differential phagocytosis and tumor growth, such as by using conditional knockout mice for Siglec-G (the mouse version of Siglec-10). In addition, all mouse experiments were performed on immunodeficient NSG mice, which lack adaptive immunity. Although the paper focuses on crosstalk between tumor cells and macrophages and made use of more simplified systems to address CD24 signalling through Singlec-10, it is increasingly clear how the adaptive immune system influences innate immune function, by means of cytokine/chemokine secretion and inhibitory/stimulatory signals (CD40/CD40L, PD-1/PD-L1, VISTA) for instance (16,41,42). The net sum of signals may ultimately alter the phenotypic outcome of myeloid cell function. Therefore, the use of murine immunocompetent models would be a valuable tool to confirm CD24’s value as a target for cancer immunotherapy.
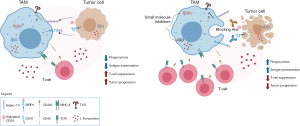
In summary, overcoming immunotherapy resistance is a major focus of research in the scientific community and identifying mechanisms in myeloid cells that could be exploited to switch their function towards an immunostimulatory state has the potential to enhance antitumor immunity and generate more effective immunotherapeutic combination strategies (Figure 1). The results presented by Barkall and collaborators propose a novel therapeutic target with particular promise for the treatment of ovarian and breast cancers, which currently lack immunotherapy options. Recent clinical successes of CAR-T cells and immune checkpoint inhibitors have led to a T-cell centric view of tumor immunity. Given that the onset and proper maintenance of T cell responses are highly dependent on the interplay between adaptive and innate immunity, harnessing the function of myeloid cells—to either overcome suppression or enhance their effector function of phagocytosis/antigen-presentation—open up new possibilities for more durable and robust tumor control with immunotherapies.
Acknowledgments
Funding: We acknowledge the funding sources of the NIH, NCI Cancer Center Support Grant P30 CA008748; NCI R01 CA056821; the Ludwig Collaborative and Swim Across America Laboratory; the Parker Institute for Cancer Immunotherapy, MSK and Breast Cancer Research Foundation.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1816). The series “Tumor Associated Macrophages in Solid Tumor: Friend or Foe” was commissioned by the editorial office without any funding or sponsorship. TM reports other from IMVAQ therapeutics, personal fees from Immuno Therapeutics, personal fees from Pfizer, grants from Bristol-Myers Squibb, grants from Surface Oncology, grants from Kyn Therapeutics, grants and personal fees from Infinity Pharmaceuticals, Inc., grants from Peregrine Pharmeceuticals, Inc., grants from Adaptive Biotechnologies, grants from Leap Therapeutics, Inc., grants from Aprea, outside the submitted work; In addition, TM has a patent Inventor on patent applications related to work on Oncolytic Viral therapy issued, a patent Alpha Virus Based Vaccine issued, a patent Neo Antigen Modeling issued, a patent CD40 pending, a patent GITR issued, a patent OX40 issued, a patent PD-1 issued, and a patent CTLA-4 issued. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med 2018;283:110-20. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin Cancer Biol 2020;65:13-27. [Crossref] [PubMed]
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015;27:462-72. [Crossref] [PubMed]
- De Vlaeminck Y, Gonzalez-Rascon A, Goyvaerts C, et al. Cancer-Associated Myeloid Regulatory Cells. Front Immunol 2016;7:113. [Crossref] [PubMed]
- De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 2016;539:443-7. [Crossref] [PubMed]
- Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature 2016;539:437-42. [Crossref] [PubMed]
- Neubert NJ, Schmittnaegel M, Bordry N, et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med 2018;10:eaan3311.
- Holmgaard RB, Brachfeld A, Gasmi B, et al. Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology 2016;5:e1151595. [Crossref] [PubMed]
- Holmgaard RB, Zamarin D, Li Y, et al. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep 2015;13:412-24. [Crossref] [PubMed]
- Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017;9:eaak9679.
- Campesato LF, Merghoub T. Antiangiogenic therapy and immune checkpoint blockade go hand in hand. Ann Transl Med 2017;5:497. [Crossref] [PubMed]
- Khalil DN, Suek N, Campesato LF, et al. In situ vaccination with defined factors overcomes T cell exhaustion in distant tumors. J Clin Invest 2019;129:3435-47. [Crossref] [PubMed]
- Suek N, Campesato LF, Merghoub T, et al. Targeted APC Activation in Cancer Immunotherapy to Enhance the Abscopal Effect. Front Immunol 2019;10:604. [Crossref] [PubMed]
- Hoves S, Ooi CH, Wolter C, et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med 2018;215:859-76. [Crossref] [PubMed]
- Demaria O, Cornen S, Daeron M, et al. Harnessing innate immunity in cancer therapy. Nature 2019;574:45-56. [Crossref] [PubMed]
- Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma. N Engl J Med 2018;379:1711-21. [Crossref] [PubMed]
- Edris B, Weiskopf K, Volkmer AK, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A 2012;109:6656-61. [Crossref] [PubMed]
- Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662-7. [Crossref] [PubMed]
- Anderson KL, Snyder KM, Ito D, et al. Evolutionarily conserved resistance to phagocytosis observed in melanoma cells is insensitive to upregulation of pro-phagocytic signals and to CD47 blockade. Melanoma Res 2020;30:147-58. [Crossref] [PubMed]
- Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138:286-99. [Crossref] [PubMed]
- Liu X, Pu Y, Cron K, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015;21:1209-15. [Crossref] [PubMed]
- Tseng D, Volkmer JP, Willingham SB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 2013;110:11103-8. [Crossref] [PubMed]
- Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019;572:392-6. [Crossref] [PubMed]
- Chen GY, Tang J, Zheng P, et al. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 2009;323:1722-5. [Crossref] [PubMed]
- Toubai T, Hou G, Mathewson N, et al. Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice. Blood 2014;123:3512-23. [Crossref] [PubMed]
- Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene 2016;35:5931-41. [Crossref] [PubMed]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 2013;38:209-23. [Crossref] [PubMed]
- Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012;12:860-75. [Crossref] [PubMed]
- Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010;11:1014-22. [Crossref] [PubMed]
- Campesato LF, Silva APM, Cordeiro L, et al. High IL-1R8 expression in breast tumors promotes tumor growth and contributes to impaired antitumor immunity. Oncotarget 2017;8:49470-83. [Crossref] [PubMed]
- Molgora M, Bonavita E, Ponzetta A, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 2017;551:110-4. [Crossref] [PubMed]
- Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis 2013;4:e688. [Crossref] [PubMed]
- Wang YJ, Fletcher R, Yu J, et al. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis 2018;5:194-203. [Crossref] [PubMed]
- Rodrigues E, Macauley MS. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers (Basel) 2018;10:207. [Crossref] [PubMed]
- Szabo R, Skropeta D. Advancement of Sialyltransferase Inhibitors: Therapeutic Challenges and Opportunities. Med Res Rev 2017;37:219-70. [Crossref] [PubMed]
- Haas Q, Simillion C, von Gunten S. A Cartography of Siglecs and Sialyltransferases in Gynecologic Malignancies: Is There a Road Towards a Sweet Future? Front Oncol 2018;8:68. [Crossref] [PubMed]
- Kohnz RA, Roberts LS, DeTomaso D, et al. Protein Sialylation Regulates a Gene Expression Signature that Promotes Breast Cancer Cell Pathogenicity. ACS Chem Biol 2016;11:2131-9. [Crossref] [PubMed]
- Almaraz RT, Tian Y, Bhattarcharya R, et al. Metabolic flux increases glycoprotein sialylation: implications for cell adhesion and cancer metastasis. Mol Cell Proteomics 2012;11:M112 017558.
- Strauss L, Mahmoud MAA, Weaver JD, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 2020;5:eaay1863.
- Johnston RJ, Su LJ, Pinckney J, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 2019;574:565-70. [Crossref] [PubMed]


