Is fibrinogen plasma level a risk factor for the first 24-hour death of medically treated acute type A aortic dissection patients?
Introduction
Acute type A aortic dissection (ATAAD) is a life-threatening disease with extremely high mortality (1). Without surgical intervention, ATAAD patients have an hourly mortality rate of 1% to 2% after occurrence (2). Therefore, ATAAD should be urgently evaluated for emergency surgical repair (3). Although there is a sound scientific explanation for the better outcomes in patients who undergo early surgery (4), it is also generally accepted that patients who survive the first 48 hours self-select themselves toward better outcomes following surgical repair (5,6). Despite advances in surgical treatment, such as hypothermic circulatory arrest, the frozen elephant trunk technique and retrograde cerebral perfusion or selective antegrade cerebral perfusion, performing an emergency surgery for ATAAD can sometimes be challenging for the entire surgical team. A recent study showed that perioperative mortality was much higher among those who underwent emergency surgery during night time (7). Hence, a risk stratification system is needed to inform clinical decisions by the surgeons better to improve the prognosis of ATAAD patients.
Some ATAAD patients in our center did not receive surgery for other reasons. Firstly, a few families of patients firmly refused surgical intervention due to high perioperative mortality. Additionally, several patients could not afford the medical cost of the surgery through the present health insurance system in China (8-10). This unfortunate reality offers a unique opportunity for cardiac surgeons to observe the development of ATAAD without surgical intervention. Therefore, the present study assessed the risk factors affecting the outcome of nonsurgical treatment in patients with ATAAD. We hope that, with this work, we can better understand which risk factors affect the surgeon’s decision of the optimal time to perform surgery for this population.
Fibrinogen is a very common substance in coagulation system. It is closely related to the adverse prognosis of heart disease. However, whether fibrinogen could provide valuable clinical prediction information for adverse outcome in ATAAD is still lacking systematic investigations. This present study was undertaken therefore to assess the plasma levels of fibrinogen in ATAAD and indicated its value in predicting risk of 24-hour death.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5466).
Methods
Patient selection
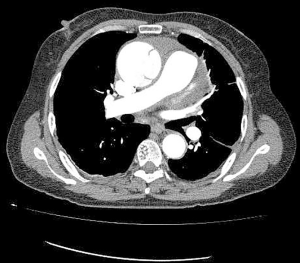
Data collected from the database “A study of the prediction model for and interventions in Acute Aortic Syndrome (ChiCTR1900022637)” were included for the analysis. The study protocol was approved by the Ethics Committee of Anzhen Hospital (Institutional Review Board File 2014019) and adhered to the principles outlined in the Declaration of Helsinki (as revised in 2013) (11). Patients were consecutively recruited if they agreed to provide informed consent. In this study, all patients were confirmed to have type A aortic dissection (TAAD) through computed tomography angiography (CTA) (Figure 1). Experienced clinical radiologists evaluated CTAs to define the presence and extent of the dissection flap and detect areas of malperfusion. From January 2009 to January 2018, 2,379 patients with TAAD were admitted to Beijing Anzhen Hospital, Capital Medical University. The patients with contraindication of surgical repairs were looked after by the emergency team of the hospital. All the patients admitted to our surgical team were suitable candidates for the surgery; even some of them had malperfusion syndrome. After excluding patients who received surgical intervention or with chronic type A dissection, 243 patients participated in the final analysis (Figure 2). Although a large part of ATAAD patients received medical management for more than 24 hours before surgery in our hospital, they were still excluded from this study for the requirement of the Cox proportional-hazards regression model. All patients received medical therapy following the guideline; the blood pressure was controlled within the normal range. Malperfusion was defined as patients with signs and symptoms of inadequate blood flow to the end organs using a combination of physical examination, clinical history, laboratory values, and radiographic studies according to the guidelines and was classified into four categories: limb, myocardial, mesenteric, and cerebral.
Data collection
The following data were assembled with the use of a standardized format from the database of “A study of the prediction model for and interventions in Acute Aortic Syndrome (ChiCTR1900022637)”: patient demographics, clinical presentations, imaging examinations, and biochemical tests. Blood samples were obtained within 6 hours of admission and were sent to the clinical laboratory within minutes of collection, and the blood tests included creatinine, platelet, fibrinogen, D-dimer, troponin I and prothrombin time. Fibrinogen was assessed by using thrombin to convert fibrinogen to fibrin. GE (USA) vivid7 and E9 ultrasound system (M3S) were used for 2D and Doppler echocardiographic studies. The patients were examined in the supine position by echocardiography. The parameters obtained included aortic sinus diameter, ascending aortic diameter, left atrial diameter, left ventricular end-systolic diameter, left ventricular end-diastolic diameter, ejection fraction, pericardial effusion, and valvular regurgitation. The 24-hour mortality, which was defined as the death from the actual onset symptoms of ATAAD, was used as the primary endpoint as the International Registry of Acute Aortic Dissection (IRAD) revealed a slightly lower risk of death after 24 hours (12).
Statistical analysis
Continuous variables showing normal distribution were expressed as the mean ± standard deviation (SD); continuous variables showing a non-normal distribution were expressed as the median [interquartile range (IQR)]. Categorical variables were described as number (percentage). T-tests were used to assess whether the continuous variables followed a normal distribution. When variables were not distributed normally, the Wilcoxon rank-sum test was applied. Chi-square test or Fisher’s test was applied to compare categorical variates. Cox proportional-hazards regression model was used to assess the association between fibrinogen and death within 24 hours of onset and in-hospital death. Based on the recommendations of the STROBE statement, we also showed the results of the unadjusted, minimum adjustment analysis, and the results of the full adjustment analysis. We selected these confounders based on their associations with the outcomes of interest or a change in effect estimate of more than 10% (13). In addition, we used clinical cutoffs of fibrinogen plasma levels (14), and defined three categories: hypofibrinogenemia (<2 g/L), normal fibrinogen level (2–4 g/L), and hyperfibrinogenemia (>4 g/L). To clarify the possible nonlinear association between fibrinogen level and HRs 24-hour death, we used a restricted cubic spline model to determine whether there were any significant hazard changes at specific fibrinogen levels. P<0.05 was considered statistically significant (two-sided). All analyses were completed by the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA).
Results
Characteristics of the patients
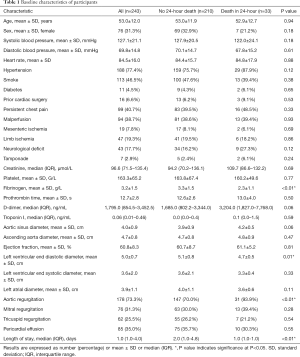
A total of 243 patients took part in the final analysis, all of whom denied the surgery for non-medical factors. The total in-hospital mortality rate was 37.9% (92/243) among this population. A total of 13.6% of patients (33/243) died within 24 hours of onset, the 24-hour mortality rate in fibrinogen <2 g/L group, fibrinogen 2–4 group and fibrinogen >4 g/L group was 22.6% (14/62), 15.5% (17/110) and 2.8% (2/71) respectively. Causes of death included aortic rupture and cardiac tamponade. The remaining patients were discharged after at least 24 hours and continued to receive medical treatment. The details of the clinical features of ATAAD patients upon admission to the hospital are presented in Table 1.
Full table
Univariate analysis
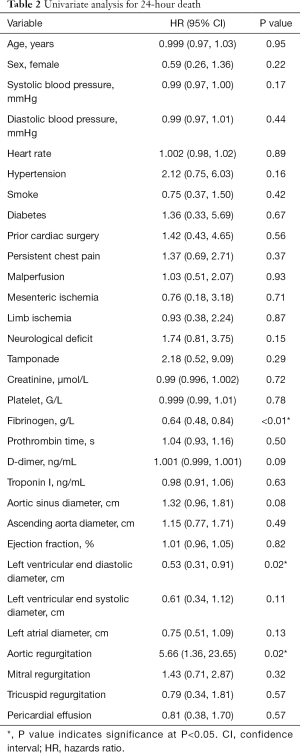
The univariate analysis results are presented in Table 2. The results of univariate analysis showed that the fibrinogen plasma level (HR, 0.64; 95% CI, 0.48–0.84; P<0.01), left ventricular end diastolic diameter (HR, 0.53; 95% CI, 0.31–0.91; P=0.02), and aortic regurgitation (HR, 5.66; 95% CI, 1.36–23.65; P=0.02) were significantly correlated with death within 24 hours.
Full table
The relationship between fibrinogen level and the first 24-hour mortality
The Cox proportional-hazards regression model was performed to evaluate the association between the fibrinogen level and death. In the crude model, the 24-hour mortality rate decreased as the fibrinogen level increased (HR, 0.64; 95% CI, 0.48–0.84; P<0.01). In the minimally adjusted model (adjusted age, sex), a similar relationship was observed (HR, 0.64; 95% CI, 0.49–0.85; P<0.01). Adjustment of all the indicators (age, sex, D-dimer level, Troponin I, and aortic sinus diameter) that might affect death also yield a similar result (HR, 0.64; 95% CI, 0.47–0.87; P<0.01) (Table 3). However, no statistically significant association was found between the fibrinogen plasma level and in-hospital mortality in all models (Table 4). For the sensitivity analysis, we divided the patients into three groups according to the clinical cutoffs. Patients with hyperfibrinogenemia (fibrinogen >4 g/L) had a lower 24-hour mortality compared with patients presented with hypofibrinogenemia (fibrinogen <2 g/L) (HR, 0.14; 95% CI, 0.03–0.62; P<0.01) (Table 3). We also divided the patients into two groups using a fibrinogen =2.0 or 4.0 g/L level as a cutoff value (Table 3). The result demonstrated that the risk of 24-hour mortality in the fibrinogen level of ≤4.0 g/L group was 4.92 times higher than that in the fibrinogen level of >4.0 g/L group (HR, 5.92; 95% CI, 1.40–25.08; P=0.02). Meanwhile, there were no significant associations observed between the fibrinogen <2.0 g/L group and fibrinogen ≥2.0 g/L groups after adjustment for all the covariates (HR, 1.87; 95% CI, 0.88–3.96; P=0.10).
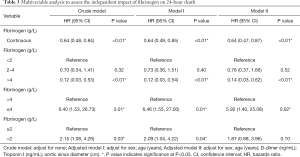
Full table

Full table
Analyses of nonlinear relationships
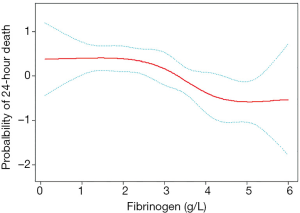
A restricted cubic spline analysis was conducted to address the nonlinearity of the relation between fibrinogen and 24-hour death (Figure 3). The adjusted smooth curve fitting showed the adverse relationship between the fibrinogen plasma level and the first 24-hour death (P<0.05). The solid red line is the estimated values, and the dashed blue line is the 95% CI.
Discussion
Without surgical intervention, TAAD patients have an hourly mortality rate of 1% to 2% after symptoms appear (2). Thus, the superiority of emergency surgery over conservative treatment has been recommended by the guidelines (1). Although there has been a significant decline in the in-hospital surgical mortality rate of patients presenting with ATAAD with continuous improvement in surgical techniques, emergency surgical repair still has a high mortality rate (3.09% to 30%) (15,16). On the other hand, patients who survive the first 48 hours self-select themselves toward better outcomes following surgical repair (5,6). Some surgeons argued that patients with malperfusion syndrome were better suited for elective surgery because they had very high perioperative mortality (17), and patients who survived for more than 48 hours after onset were better suited for initial medical management with the possibility of later operative treatment (18). When considering any patient with ATAAD, the primary question guiding management should be whether the risk of rupture outweighs the risk of surgical repair. Patients may die due to sudden aortic rupture or organ ischemia during the waiting period. Added investigations are needed to confirm that postponing surgery in patients with organ ischemia is safe and reasonable. Moreover, the emergency ATAAD operation still presents a considerable challenge for the whole surgical team—from surgeons and anesthetists to perfusionists and nurses (19). Further, the higher mortality rate in patients undergoing nighttime surgical procedures for ATAAD was reported because the number of hospital staff at night was limited, and surgeons prefer to perform surgery the next day for patients who arrive at the hospital during the night (7). Thus, surgeons need to choose the best intervention time for ATAAD, even if the guidelines indicate that ATAAD should receive emergency surgical repair (3).
Although all the patients admitted to our surgical center were suitable candidates for the surgery, there were still some ATAAD patients at our center who only received medical therapy finally. A small proportion of them firmly refused surgical intervention due to the high perioperative mortality and risk of repeat surgery on the distal aorta. Most of these patients could not afford the cost of the surgery. Although the government health expenditure (GHE) in China has increased by over 10% during the past several years, the mean reimbursement rate of inpatient care among respondents older than 15 years is 41.19% across the entire country and only 34.58% in rural areas under the present health insurance system (8,10). The patients must pay the remaining medical costs (approximately 60%) before they are admitted to the inpatient ward. The total cost of ATAAD surgery at our center is 30,000 US dollars, which means that patients must pay 18,000 US dollars before admission. Indeed, there are still quite a lot of patients with ATAAD who have no chance to get surgical treatment for some non-medical reasons. The ratio of medical treatment in the present study is 10.2% (243/2,379). Given the patients with contraindication in our emergency department, this ration would be even higher. However, this provided a unique opportunity to gain experience about the risk factors affecting the outcome of medical treatment in patients with ATAAD. More importantly, we can use this situation to better understand which patients must undergo surgery immediately, and patients’ surgery can tolerate a delay, thereby allowing the surgeons more time to prepare.
The TAAD was further divided into four phases according to the Kaplan-Meier survival curve: hyperacute (0–24 hours), acute (2–7 days), subacute (8–30 days), and chronic (30 days) (20). Patients who die in the first 24 hours are often the most critically ill patients, and most die while waiting for surgery or during referrals. After 24 hours, there is a slightly lower risk of death (12), which continues to decrease between days 5 and 30 at a rate of 1% per day (21). It is much more essential to decide the best surgical intervention time during the first 24 hours. Therefore, we used the first 24-hour mortality as the primary endpoint.
In this study, the mortality of hyperacute TAAD was only 13.6%, and those treated medically had an overall in-hospital mortality of 37.9%, which was lower than the previous outcome (20,22). This finding was not unexpected, in that a shorter length of stay was found in other studies (22). Also, some surgeons hold that the presence or absence of malperfusion is the primary determinant of outcomes in patients undergoing repair of aortic dissection (6). Nevertheless, the results revealed that malperfusion syndrome (38.7%) was not associated with death within the first 24 hours. We speculate that the reason for this outcome is because a substantial proportion of patients with hyperacute TAAD, including patients complicated with malperfusion syndrome, had undergone surgery during the first 24 hours.
We note that the fibrinogen plasma level was independently associated with all-cause mortality within 24 hours of ATAAD onset without surgical intervention. In later analysis, a fibrinogen level of ≤4.0 g/L was significantly associated with a 3.5-fold higher mortality rate in ATAAD patients after adjusting for all confounding factors. It would be helpful to show risk factors for adverse outcomes to achieve better outcomes in patients with ATAAD. Fibrinogen is synthesized and secreted by the liver, which takes part in thrombosis. The usual range of fibrinogen in the human body is 2 to 4 g/L (14). In the final stage of coagulation. Soluble fibrinogen transforms into insoluble fibrin, which makes blood coagulate. When the level of fibrinogen increases, the blood is in a high coagulation state, blood flow slows down, and blood viscosity increases, increasing the susceptibility to thrombosis (23). Fibrinogen is a significant independent risk factor for coronary heart disease, stroke, and other vascular events (24). The balance of coagulation and the fibrinolysis system is also disrupted in cases of aortic dissection. False lumen formation causes subendothelial components to be exposed and tissue factors to be released, which results in the activation of coagulation and the fibrinolysis system. As a result, thrombin is produced, and a large amount of fibrinogen is consumed. Previous studies have consistently shown the same changes in the hemostatic system in patients with ATAAD (25,26). When the fibrinogen plasma level decreases, the viscosity of the blood also decreases, causing blood to flow more quickly through the vessels, and thus, the aorta may suffer from the more significant impact on the bloodstream, thereby increasing the risk of aortic dissection rupture. An early study reported that fluid extravasation is a risk factor for preoperative mortality in ATAAD (27). Moreover, favorable outcomes with initial medical treatment for ATAAD with a thrombosed false lumen of the ascending aorta have been reported in Korea and Japan (28,29). This suggests that the absence of a moving bloodstream in the ascending aortic false lumen may significantly reduce the risk of adverse outcomes.
The causes of aortic rupture are multi-factorial. Morphology or bloodstream of the false lumen, the thickness of the aortic wall or adventitia, thrombosis of the false lumen, or hemodynamics have influenced. However, unlike earlier studies, we focused on laboratory indicators to explain the effect of hemodynamics on adverse events in aortic dissection. Our study proved the protective effect of hyperfibrinogenemia (30) (fibrinogen plasma levels >4.0 g/L) on 24-hour mortality in patients who present with ATAAD. These results are unique because the literature that reported the association between hyperfibrinogenemia and cardiovascular disease were unable to demonstrate this effect in aortic disease. Although the occurrence of aortic dissection may lead to the destruction of the coagulation and fibrinolysis system, some patients with suitable compensation mechanisms can produce a large amount of fibrinogen, which manifests as hyperfibrinogenemia in a stressed or inflammatory state, thereby preventing dissection rupture. Nevertheless, it is interesting to note that normal fibrinogen levels (2 to 4 g/L) are not enough to protect against aortic dissection rupture. These results justify recommendations for surgical intervention without any hesitation in patients with fibrinogen plasma levels of ≤4.0 g/L to avoid adverse outcomes, even though some other risks may be present.
Limitations
First, it is impossible to determine the number of patients who died before arrival. Thus, we are unable to determine the exact mortality rates for the conditions described. Secondly, our data are based on a large number of ATAAD patients who received medical treatment, but this is a retrospective cohort study and could only support an association but not a causal relationship between fibrinogen and 24-hour mortality. And the absolute number of 33 events may not be enough to allow adequate multivariable competing analyses. About 90% of patients received surgical intervention, and selection bias might exist in our study result. However, the patient selection was non-medical, such as patient and family’s refusal or economic factor, so medial reasons did not cause this bias.
Further, studies with a more international, multicenter, and more extensive data set are still needed to verify the study results. Finally, the finding of the protective effect of hyperfibrinogenemia on the first 24-hour mortality suggests that ATAAD patients without surgery might receive help from the administration of fibrinogen. Thus, the possible medical treatment found by the present study must be further explored in future studies. Despite these limitations, this study provides unique information from a large aortic center with nonsurgical treatment data on ATAAD.
Conclusions
In conclusion, fibrinogen level ≤4.0 g/L was an independent risk factor for the first 24-hour mortality of medical treatment in patients with ATAAD. ATAAD patients with a fibrinogen plasma level of >4.0 g/L had lower first 24-hour mortality when treated medically. For these patients, surgeons might have more time to prepare for the operation. On the other hand, patients with a fibrinogen plasma level of ≤4.0 g/L are more likely to die without surgery in the first 24 hours. They should receive the immediate surgical intervention, despite some other high-risk factors. If they cannot receive the urgent surgery for several reasons, the administration of fibrinogen might be helpful to reduce the first 24-hour mortality. However, these conclusions still require further validation with larger samples and added data. Future trials on the association between fibrinogen and aortic diseases should also consider blood rheology.
Acknowledgments
Funding: This study was supported by the National Key R&D Program of China (No. 2017YFC1308000), National Natural Science Foundation of China (No. 81800404), Capital Health Development Research Project (No. 2018-4-2068), Beijing Municipal Administration of Hospitals’ Youth Program (No. QML20180601), Foundation of Beijing Outstanding Young Talent Training Program (No. 2017000021469G254), and Beijing Lab for Cardiovascular Precision Medicine (No. PXM2017_014226_000037).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5466
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5466
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5466). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Committee of Anzhen Hospital (Institutional Review Board File 2014019) and adhered to the principles outlined in the Declaration of Helsinki (as revised in 2013). Patients were consecutively recruited if they agreed to provide informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800-11. [Crossref] [PubMed]
- Wang D, Wang ZY, Wang JF, et al. Values of aortic dissection detection risk score combined with ascending aorta diameter >40 mm for the early identification of type A acute aortic dissection. J Thorac Dis 2018;10:1815-24. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Ehrlich MP, Ergin MA, McCullough JN, et al. Results of immediate surgical treatment of all acute type A dissections. Circulation 2000;102:III248-52. [Crossref] [PubMed]
- Scholl FG, Coady MA, Davies R, et al. Interval or permanent nonoperative management of acute type A aortic dissection. Arch Surg 1999;134:402-5; discussion 405-6. [Crossref] [PubMed]
- Narayan P, Rogers CA, Benedetto U, et al. Malperfusion rather than merely timing of operative repair determines early and late outcome in type A aortic dissection. J Thorac Cardiovasc Surg 2017;154:81-6. [Crossref] [PubMed]
- Qiu J, Zhang L, Luo XJ, et al. Higher Mortality in Patients Undergoing Nighttime Surgical Procedures for Acute Type A Aortic Dissection. Ann Thorac Surg 2018;106:1164-70. [Crossref] [PubMed]
- Yip W, Fu H, Chen AT, et al. 10 years of health-care reform in China: progress and gaps in Universal Health Coverage. Lancet 2019;394:1192-204. [Crossref] [PubMed]
- Meng Q, Fang H, Liu X, et al. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet 2015;386:1484-92. [Crossref] [PubMed]
- Li X, Lu J, Hu S, et al. The primary health-care system in China. Lancet 2017;390:2584-94. [Crossref] [PubMed]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014;81:14-8. [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Yoon BW, Bae HJ, Hong KS, et al. Phenylpropanolamine contained in cold remedies and risk of hemorrhagic stroke. Neurology 2007;68:146-9. [Crossref] [PubMed]
- Erdoes G, Koster A, Meesters MI, et al. The role of fibrinogen and fibrinogen concentrate in cardiac surgery: an international consensus statement from the Haemostasis and Transfusion Scientific Subcommittee of the European Association of Cardiothoracic Anaesthesiology. Anaesthesia 2019;74:1589-600. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, Lack of Evidence, Controversy, and Debate in the Provision and Performance of the Surgery of Acute Type A Aortic Dissection. J Am Coll Cardiol 2011;58:2455-74. [Crossref] [PubMed]
- Yang B, Norton EL, Rosati CM, et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: A 20-year experience. J Thorac Cardiovasc Surg 2019;158:675-87.e4. [Crossref] [PubMed]
- Davies RR, Coe MP, Mandapati D, et al. Thoracic Surgery Directors Association Award. What is the optimal management of late-presenting survivors of acute type A aortic dissection. Ann Thorac Surg 2007;83:1593-601. [Crossref] [PubMed]
- Goldfinger JZ, Halperin JL, Marin ML, et al. Thoracic Aortic Aneurysm and Dissection. J Am Coll Cardiol 2014;64:1725-39. [Crossref] [PubMed]
- Booher AM, Isselbacher EM, Nienaber CA, et al. The IRAD Classification System for Characterizing Survival after Aortic Dissection. Am J Med 2013;126:730.e19-24. [Crossref] [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114:I350-6. [Crossref] [PubMed]
- Pacini D, Di ML, Fortuna D, et al. Acute aortic dissection: epidemiology and outcomes. Int J Cardiol 2013;167:2806-12. [Crossref] [PubMed]
- Sweetnam PM, Thomas HF, Yarnell JW, et al. Fibrinogen, viscosity and the 10-year incidence of ischaemic heart disease. Eur Heart J 1996;17:1814-20. [Crossref] [PubMed]
- Fibrinogen Studies Collaboration, Danesh J, Lewington S, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 2005;294:1799-809. [PubMed]
- Guan XL, Wang XL, Liu YY, et al. Changes in the Hemostatic System of Patients With Acute Aortic Dissection Undergoing Aortic Arch Surgery. Ann Thorac Surg 2016;101:945-51. [Crossref] [PubMed]
- Paparella D, Rotunno C, Guida P, et al. Hemostasis alterations in patients with acute aortic dissection. Ann Thorac Surg 2011;91:1364-9. [Crossref] [PubMed]
- Erbel R, Oelert H, Meyer J, et al. Effect of medical and surgical therapy on aortic dissection evaluated by transesophageal echocardiography. Implications for prognosis and therapy. The European Cooperative Study Group on Echocardiography. Circulation 1993;87:1604-15. [Crossref] [PubMed]
- Song JK, Yim J, Ahn JM, et al. Outcomes of patients with acute type a aortic intramural hematoma. Circulation 2009;120:2046-52. [Crossref] [PubMed]
- Kitai T, Kaji S, Yamamuro A, et al. Clinical outcomes of medical therapy and timely operation in initially diagnosed type A aortic intramural hematoma: a 20-year experience. Circulation 2009;120:S292-8. [Crossref] [PubMed]
- Mauriello A, Sangiorgi G, Palmieri G, et al. Hyperfibrinogenemia is associated with specific histocytological composition and complications of atherosclerotic carotid plaques in patients affected by transient ischemic attacks. Circulation 2000;101:744-50. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)