
Nonalcoholic fatty liver disease impacts the control of the international normalized ratio in patients with atrial fibrillation
Introduction
Anticoagulant therapy is an effective method of preventing ischemic stroke in patients with atrial fibrillation (AF) (1). Although a number of novel direct acting oral anticoagulants (OACs) have been approved, warfarin has been the worldwide primary OAC for the past 50 years, and is currently prescribed to nearly 35% of patients with AF (2). Based on recent data from a cross-national survey in China, among patients with ischemic stroke and a history of AF who had taken OACs, 98.2% had taken warfarin, and there was large underuse of OACs (3). Therapeutic anticoagulation is defined as an international normalized ratio (INR) of 2.0–3.0; the warfarin dose required to achieve this for patients can vary more than 40-fold, ranging from 0.5–20 mg/day (4). This dose variability is associated with clinical factors such as ethnicity, age, body-mass index (BMI), body surface area, smoking, drug-drug/dietary interactions, genetic variants, and also concomitant conditions, including impaired nutrition, congestive heart failure, pharmacogenetic-guided dosing of coumarin anticoagulants, and liver diseases (5). Some of these clinical factors may also affect INR. For example, a study include 4202 patients found that the risk of hemorrhage increased sharply and INR was influenced with advanced age; Polymorphisms in cytochrome P450 2C9 (CYP2C9) and VKORC1 influence patients’ INR; Interactions between warfarin and certain commonly used drugs and drug families (mainly anti-infective agents, lipid-lowering drugs, NSAIDs including COX-2 selective NSAIDs, selective serotonin reuptake inhibitors, amiodarone, omeprazole, fluorouracil, and cimetidine) are also cause for concern of the influence on INR (6-8) .
Nonalcoholic fatty liver disease (NAFLD) is one of the most widespread liver diseases in the world, with prevalence estimates ranging from 6–35%. Recently, increasing evidence has indicated that NAFLD is linked with multiple types of cardiovascular disease (9). Also, NAFLD appears to be increasing in frequency and is related with higher adverse cardiovascular outcomes in AF patients (10,11). It has been reported that AF patients who develop NAFLD have relatively lower levels of INR, compared with those who do not (12). Evidence of mechanism suggested that impaired liver function influenced the metabolism of warfarin (13).
It is unclear whether the therapeutic effect of warfarin in AF patients with NAFLD, but normal liver function, differs from the effect in AF patients without NAFLD. So far, only a few studies have focused on the complication of NAFLD affecting warfarin dosage requirements. As they have reported, patients with NAFLD are often accompanied by a decline in liver function, and an increased activity of some circulating coagulation factors (FVIII, FIX, FXI and FXII) has been found in patients of NAFLD, while the increase of FIX may attenuate the function of warfarin. Besides, a procoagulant imbalance has been observed across the spectrum of NAFLD histological severity progressing from simple steatosis to NASH-cirrhosis and resulting from reduced protein C, which may influence the effect of warfarin (14). Furthermore, impaired liver may influence the metabolism of warfarin thus affecting warfarin dosage requirements. Therefore, in this study, we aimed to compare the INR control in warfarin-treated AF and NAFLD patients with normal liver function to that of patients without NAFLD.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5387).
Methods
We performed a cross-sectional study including all AF patients who visited the Xinhua Hospital from Jan 2016 to Dec 2018 and received warfarin therapy. The data were extracted from electronic medical records in the Xinhua Hospital. In this way, we identified 600 AF patients aged 28–94 years with normal liver function who were taking warfarin regularly; of these, 172 had NAFLD, and 428 did not.
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine Chongming Branch (No. CMEC-2020-KT-31) and informed consent was taken from all the patients.
Diagnosis of AF
AF was diagnosed according to a standard 12-lead resting electrocardiograph or 24-hour Holter, during the outpatient or inpatient period of the enrolled participants. All procedures and operations were performed by experienced cardiologists.
Determination of NAFLD
In this study, NAFLD was diagnosed according to the current guidelines from the American Association for the Study of Liver Diseases (AASLD) (15), National Institute for Health and Care Excellence (NICE) (16), and the European associations for the study of the liver, diabetes, and obesity (EASL-EASD-EASO) (17).
A Hewlett-Packard ultrasound color system, Sonos 500 (HP, Andover, Mass, USA), was used for the echocardiographic examinations. M-mode measurements were taken under 2-D guidance in accordance with the recommendations of the American Society of Echocardiography (18), if all following criteria were met: (I) ultrasound identification of hepatic steatosis, assessed by professional radiologists with at least 10 years’ experience of abdominal ultrasound examination; and (II) exclusion of secondary known causes of chronic liver disease, including excessive alcohol intake (30 g/day in men and 20 g/day in women), viral infection, drugs that can injure the liver, autoimmunity, and hemochromatosis (17).
Warfarin treatment
AF patients with NAFLD were treated with warfarin in accordance with the current American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations (19). Thus, warfarin was prescribed as an oral anticoagulant (OAC) for patients with nonvalvular AF who had a CHA2DS2-VASc score ≥2.
Covariates
From the electronic medical records, we extracted the data on demographic information, physical examination, history and comorbidity, concomitants, and laboratory tests. All data were checked and validated, if there were any outliers, we manually checked the original dataset.
All patient blood samples used for laboratory tests were drawn after an overnight fast. Concentrations of glutamate-transpeptidase (GPT) and glutamic-oxalacetic transaminase (GOT), along with INR and platelet count in blood were measured as part of routine clinical practice for patients with AF.
Statistical analyses
Mean and standard deviation, or median and range, were used to describe baseline characteristics, whenever appropriate. The differences in continuous and categorical variables between the patients with and without NAFLD were assessed using the student’s t-test and chi-square test, respectively. Nested multivariable linear regression models of INR, INR/warfarin dose rate, and NAFLD, with increasing numbers of covariates included, were developed. The covariates included were: Model 1: age, gender, and for INR only, warfarin dose; Model 2: as Model 1, adding current smoking status and alcohol consumption; Model 3: as Model 2, adding history of hypertension, cardiac insufficiency, coronary artery disease (CAD), myocardial infarction (MI), vascular diseases, cerebral infarction, cerebral hemorrhage, stroke, diabetes mellitus (DM), and cancer; Model 4: as Model 3, adding history of drug use.
All data analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC, USA). A two-tailed P value of <0.05 was considered to be statistically significant.
Results
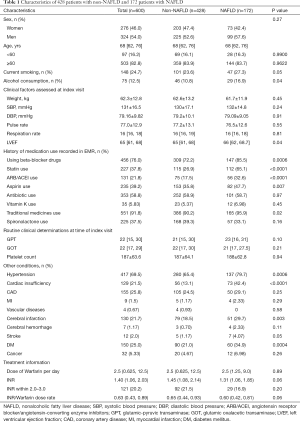
Among a total of 600 AF patients aged 28–94 years, 54% were men, and 172 patients aged 29–91 years were diagnosed with NAFLD. AF patients with NAFLD tended to have a higher proportion of left ventricular ejection fraction (LVEF), hypertension, cardiac insufficiency, cerebral infarction, stroke and DM (Table 1) . Consistent with these differences, higher proportions of AF patients with NAFLD were recorded as having been prescribed beta-blockers, statins, angiotensin-receptor blockers (ARB) or angiotensin converting enzyme inhibitors (ACEI), aspirin, or traditional medicines. In addition, higher proportions of AF patients with NAFLD were recorded as active smokers or consumers of alcohol.
The warfarin dose was very similar between patients with NAFLD [median 2.5 mg/day, interquartile range (IQR) 1.25–9.0], and without NAFLD (median 2.5, IQR 0.625–12.05). The median INR of patients with NAFLD [1.31 (P25, P75: 1.06, 1.85)] was lower than the median INR of 1.45 (P25, P75: 1.08, 2.14) for non-NAFLD patients (P=0.06). Consistent with this, the proportion of patients whose INR was within the target range of 2.0–3.0 was somewhat lower in NAFLD patients (16.9 percent) than in non-NAFLD patients (21.5 percent; P=0.20). The INR/warfarin dose rate was also slightly (0.05) lower among patients with NAFLD than in those without NAFLD (P=0.06) (Figure 1).
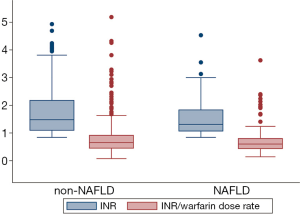
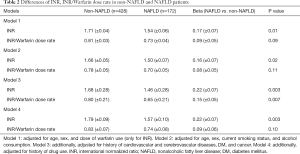
In our multivariable analyses, we found that NAFLD patients were more likely to have a lower INR than patients without NAFLD. The estimated least square means of INR were 1.54 [standard error (SE): 0.06], and 1.71 (SE: 0.04) for non-NAFLD and non-NAFLD, respectively (P=0.01) after adjustment for age, gender, and warfarin dose. After being further adjusted for current smoking status, alcohol consumption, history of cardiovascular and cerebrovascular diseases, DM, cancer, and history of drug use, the difference was not attenuated and remained statistically significant (Table 2). The INR/warfarin dose rate was slightly lower in NAFLD patients than in non-NAFLD patients, but the difference was not statistically significant in any of the models (Table 2).
Full table
Full table
Discussion
In this study, we firstly reported that the therapeutic effect of warfarin in AF patients with NAFLD is impaired despite them having normal liver function. Our findings suggested that a slightly higher or personally optimized dosage of warfarin was warranted among AF patients with NAFLD to achieve the target level INR.
The prevalence of AF in Asians is about 1%, and the burden of this disease is considerable. In China, it is estimated that there will be 5.2 million men and 3.1 million women over 60 with AF by 2050. NAFLD is another common chronic medical condition, and is defined as the accumulation of fat in >5% of hepatocytes, without competing liver disease such as viral hepatitis or autoimmune hepatitis. Meta-analysis indicated that 25.24% of the global population have NAFLD, similar to the 20% prevalence rate in China. Broadly, NAFLD is present in approximately 30–40% of men and 15–20% of women in the adult population (20,21). Recent epidemiological studies noticed that NAFLD is clinically common in AF patients. NAFLD has been observed to be significantly associated with AF, especially in patients with type 2 diabetes; AF was found to be more prevalent in hospitalized NAFLD patients with type 2 diabetes (22,23). A cohort study showed that NAFLD was associated with an increased risk of prevalent AF in a middle-aged population (24). Furthermore, a cross-sectional study showed that NAFLD is associated with an increased risk of AF in an elderly Chinese population (25). The increasing prevalence of chronic cardiometabolic diseases such as obesity, DM, metabolic syndrome, and chronic alcoholism is expected to compound the burden of NAFLD. Stroke and venous thromboembolism (VTE) are risks associated with AF, and are potential causes of morbidity in patients with NAFLD. Therefore, clinical prevention and treatment measures for the large section of society that have both AF and NAFLD, particularly the majority with normal liver function, may become a distinct strategic approach. AF patients with NAFLD have commonly been excluded from randomized clinical trials of OACs for the prevention of stroke and VTE for an extended time. Thus, the optimal anticoagulation strategy for patients with AF accompanied by NAFLD is complex and lacking definition.
Anticoagulant therapy is a key issue for the prevention of stroke in patients with AF. The oral vitamin K antagonist (VKA) warfarin is widely used to prevent stroke in patients with AF and for secondary prevention of VTE. Despite newer treatment options, such as the direct OACs, warfarin is a mainstay in anticoagulation therapy, and is still the most frequently prescribed anticoagulant. So far, the treatment persistence of non-vitamin K antagonist oral anticoagulants (NOACs) has been significantly lower than that of warfarin among Chinese patients with AF (26). Therefore, warfarin is economically applicable and efficacious for stroke prevention in AF.
Warfarin is predominantly eliminated by the liver, where it is converted to an inactive metabolite through a cytochrome P450-dependent metabolism, and does not rely on the kidney for its clearance. As a traditional VKA, warfarin indirectly inactivates clotting factors. Vitamin K is a fat-soluble vitamin discovered through its role in blood coagulation. It acts as a cofactor for the enzyme that allows the activation of vitamin K-dependent factors (II, VII, IX, X, protein C, and protein S) (27). It is noteworthy that NAFLD may disturb the metabolism of fat-soluble vitamins (A, D, and K) (28,29). Basic studies have shown that liver produces bile supporting efficient intestinal absorption of fat-soluble nutrients like vitamin A, D and K. Thus NAFLD is associated with impaired vitamin homeostasis and may lead to deficiency of these vitamins. Besides, in NAFLD patients, the function of producing retinol binding protein 4 (RBP4) and distributes vitamin A to peripheral tissue appears to be damaged and the increased body fat mass resulted in a greater volume of distribution of vitamin D in the adipose tissue compartment, and thereafter, a relatively lower plasma concentration (30). And from different angles, anti-NAFLD drugs may interfere with the absorption of many drugs (such as warfarin, amiodarone, ciclosporin and thyroxine as well as fat-soluble vitamins), affecting their bioavailability and effectiveness (31). From the perspective of potential mechanisms of drug metabolism, the influence of NAFLD on the expression and activity of CYP3A [a gene that is part of a cluster of cytochrome P450 (CYP) genes] has been studied by using animal and cell culture models, human hepatic tissues, and human subjects. NAFLD exerts an effect on phase I and phase II drug metabolizing enzymes (DMEs) (32). In addition, studies have revealed that the CYP2C9, CYP4F2, and gamma-glutamyl carboxylase (GGCX) genes have all been associated with the warfarin dose needed to achieve a common degree of anticoagulation response (33). This shows that NAFLD has a potential influence on the metabolism of warfarin via the P450 (CYP) system. There was also evidence that implied that NAFLD causes systemic inflammation that may impact warfarin metabolism. Pathogenic mediators, which release several pro-inflammatory factors into the bloodstream, including C-reactive protein, interleukin-6, tumor necrosis factor-alpha, plasminogen activator inhibitor-1, and other inflammatory cytokines, may play an important role in the metabolism of warfarin (34). All of this information led us to focus on the use of warfarin for the prevention or treatment of thromboembolic conditions in NAFLD patients with AF.
In this study, we sought to build the base of evidence for warfarin requirements in AF patients with NAFLD, by investigating factors that may affect the metabolism and required therapeutic dose of warfarin. Warfarin therapy characteristically has a narrow therapeutic index and wide interindividual response, due to clinical, demographic, and environmental factors (35). By using statistical model analysis, we adjusted for influencing factors including gender, age, body weight, cardiac function, history of cardiovascular and cerebrovascular diseases, DM, cancer, lifestyle risk factors, and history of related drug therapy; very interestingly, the results indicated an independent negative association between NAFLD and INR level after warfarin treatment. We found that AF patients with NAFLD have a tendency to lower INR levels compared to AF patients with non-NAFLD. The results in our study indicated that a higher warfarin dosage for AF patients with NAFLD, compared with non-NAFLD patients, would achieve the desirable INR target range. The current clinical guidelines do not offer specific recommendations for the use of OACs in this particular population. Therefore, an optimal anticoagulation strategy for patients with NAFLD, using warfarin, needs to be better defined, drawing on evidence from studies with larger sample sizes.
Conclusions
Among AF patients with NAFLD, but normal liver function, the therapeutic effect of warfarin might be impaired. To achieve the desirable INR target range for these patients, a stronger or personally tailored dosage of warfarin might be necessary.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (81974022 and 81500188), Shanghai Health Committee (201940206) and Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine Chongming Branch (2019YA-02).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5387
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5387
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5387). The authors report grants from National Natural Science Foundation of China, grants from Shanghai Health Committee, and grants from Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine Chongming Branch, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine (No. CMEC-2020-KT-31) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tawfik A, Bielecki JM, Krahn M, et al. Systematic review and network meta-analysis of stroke prevention treatments in patients with atrial fibrillation. Clin Pharmacol 2016;8:93-107. [Crossref] [PubMed]
- Jagadish PS, Kirolos I, Khare S, et al. Post-operative atrial fibrillation: should we anticoagulate? Ann Transl Med 2019;7:407. [Crossref] [PubMed]
- Guo J, Guan T, Fan S, et al. Underuse of Oral Anticoagulants in Patients With Ischemic Stroke and Atrial Fibrillation in China. Am J Cardiol 2018;122:2055-61. [Crossref] [PubMed]
- Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e44S-88S.
- Verhoef TI, Redekop WK, Daly AK, et al. Pharmacogenetic-guided dosing of coumarin anticoagulants: algorithms for warfarin, acenocoumarol and phenprocoumon. Br J Clin Pharmacol 2014;77:626-41. [Crossref] [PubMed]
- Torn M, Bollen WL, van der Meer FJ, et al. Risks of oral anticoagulant therapy with increasing age. Arch Intern Med 2005;165:1527-32. [Crossref] [PubMed]
- Shendre A, Parmar GM, Dillon C, et al. Influence of Age on Warfarin Dose, Anticoagulation Control, and Risk of Hemorrhage. Pharmacotherapy 2018;38:588-96. [Crossref] [PubMed]
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-106. [Crossref] [PubMed]
- Mantovani A, Ballestri S, Lonardo A, et al. Cardiovascular Disease and Myocardial Abnormalities in Nonalcoholic Fatty Liver Disease. Dig Dis Sci 2016;61:1246-67. [Crossref] [PubMed]
- Valbusa F, Bonapace S, Grillo C, et al. Nonalcoholic Fatty Liver Disease Is Associated With Higher 1-year All-Cause Rehospitalization Rates in Patients Admitted for Acute Heart Failure. Medicine (Baltimore) 2016;95:e2760. [Crossref] [PubMed]
- Ballestri S, Lonardo A, Bonapace S, et al. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol 2014;20:1724-45. [Crossref] [PubMed]
- Denkmayr L, Feldman A, Stechemesser L, et al. Lean Patients with Non-Alcoholic Fatty Liver Disease Have a Severe Histological Phenotype Similar to Obese Patients. J Clin Med 2018;7:562. [Crossref] [PubMed]
- Wang CL, Wu VC, Kuo CF, et al. Efficacy and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients With Impaired Liver Function: A Retrospective Cohort Study. J Am Heart Assoc 2018;7:e009263. [Crossref] [PubMed]
- Kotronen A, Joutsi-Korhonen L, Sevastianova K, et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int 2011;31:176-83. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol 2012;107:811-26. [Crossref] [PubMed]
- National Guideline Centre (UK). Non-Alcoholic Fatty Liver Disease: Assessment and Management. London: National Institute for Health and Care Excellence (UK); 2016.
- European Association for the Study of the L. European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Zhang JZ, Cai JJ, Yu Y, et al. Nonalcoholic Fatty Liver Disease: An Update on the Diagnosis. Gene Expr 2019;19:187-98. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-32. [Crossref] [PubMed]
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. [Crossref] [PubMed]
- Li Z, Xue J, Chen P, et al. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol 2014;29:42-51. [Crossref] [PubMed]
- Targher G, Mantovani A, Pichiri I, et al. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci (Lond) 2013;125:301-9. [Crossref] [PubMed]
- Mantovani A, Dauriz M, Sandri D, et al. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int 2019;39:758-69. [Crossref] [PubMed]
- Karajamaki AJ, Patsi OP, Savolainen M, et al. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study). PLoS One 2015;10:e0142937. [Crossref] [PubMed]
- Zhang Y, Li P, Miao M, et al. Nonalcoholic Fatty Liver Disease Is Associated with Increased Atrial Fibrillation Risk in an Elderly Chinese Population: A Cross-Sectional Study. Biomed Res Int 2018;2018:5628749.
- Liu C, Du X, Jiang C, et al. Long-Term Persistence with Newly-Initiated Warfarin or Non-VKA Oral Anticoagulant (NOAC) in Patients with Non-Valvular Atrial Fibrillation: Insights from the Prospective China-AF Registry. Med Sci Monit 2019;25:2649-57. [Crossref] [PubMed]
- Alisi L, Cao R, De Angelis C, et al. The Relationships Between Vitamin K and Cognition: A Review of Current Evidence. Front Neurol 2019;10:239. [Crossref] [PubMed]
- Saeed A, Dullaart RPF, Schreuder T, et al. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2018;10:29. [Crossref] [PubMed]
- Khavkin AI, Komarova ON. Malabsorption Fat-Soluble Vitamins and Prospects of Their Use in Liver Diseases. Eksp Klin Gastroenterol 2016.86-94. [PubMed]
- Liu S, Liu Y, Wan B, et al. Association between Vitamin D Status and Non-Alcoholic Fatty Liver Disease: A Population-Based Study. J Nutr Sci Vitaminol (Tokyo) 2019;65:303-8. [Crossref] [PubMed]
- Filippatos TD, Derdemezis CS, Gazi IF, et al. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf 2008;31:53-65. [Crossref] [PubMed]
- Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev 2017;49:197-211. [Crossref] [PubMed]
- Henderson LM, Robinson RF, Ray L, et al. VKORC1 and Novel CYP2C9 Variation Predict Warfarin Response in Alaska Native and American Indian People. Clin Transl Sci 2019;12:312-20. [Crossref] [PubMed]
- Scott SA, Lubitz SA. Warfarin pharmacogenetic trials: is there a future for pharmacogenetic-guided dosing? Pharmacogenomics 2014;15:719-22. [Crossref] [PubMed]
- Zirlik A, Bode C. Vitamin K antagonists: relative strengths and weaknesses vs. direct oral anticoagulants for stroke prevention in patients with atrial fibrillation. J Thromb Thrombolysis 2017;43:365-79. [Crossref] [PubMed]
(English Language Editor: J. Jones)


