
The influences of TGF-β1 upon the human adenocarcinoma cell of lung A549 and cellular immunity
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% to 90% of all lung cancer cases, which is the most common cause of cancer-related deaths worldwide (1). Under modern cancer treatment, the 5-year survival rate for all stages of lung cancer is about 16%. Tumor production of prostaglandin (PGs), interleukin (IL)-10, vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β and other factors, can directly or indirectly suppress the immune response, blocking immunotherapy (2). Studies have shown that (3) the production of a large number of immunosuppressive factor tumor-derived immune-suppressive factor (TDSF) in patients with malignant tumors is one of the leading causes of immune dysfunction. Transform growth factor-beta 1 (TGF-β1) secreted by tumor cells is a significant group of immune down-regulating factors (4). At present, there are few studies on TGF-β1 and cellular immunity. The purpose of this study was to investigate the effects of co-culture of human lung adenocarcinoma cell line A549 with peripheral blood mononuclear cells (PBMCs) from patients with NSCLC at different levels of TGF-β1 on T cell immunity and A549 cell line. In order to further reveal the inhibition of varying levels of TGF-β1 on the immune system of patients with lung cancer, and to provide a reference for the treatment of low immunity in patients with lung cancer.
Methods
General information
From October 2011 to December 2011, 20 inpatients with NSCLC confirmed by pathology in the Third Affiliated Hospital of Kunming Medical University were selected. Peripheral venous blood 11 mL was taken from each patient. One mL blood samples were used to detect the purity of immune factors and PBMCs in peripheral blood. Selection criteria: (I) the patients with NSCLC were confirmed by clinicopathology, with no limit of sex, clinical stage, and no operation within 3 months; (II) age 30 to 75 years old; (III) after clinical examination, the function of the heart, liver, brain, and kidney was normal; (IV) no patients received chemotherapy, radiotherapy, or biological immunotherapy within 6 months. Exclusion standard: (I) patients complicated with primary immune system diseases; (II) complicated with diabetes mellitus; (III) patients complicated with malignant tumors of other systems; (IV) patients with end-stage cachexia of lung cancer; (V) those who had used hormonal drugs and immune preparations in the past 6 months. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee approved this study of the third affiliated Hospital of Kunming Medical University. All the subjects had informed consent of the trial and signed the informed consent form.
Main experimental instruments and materials
Human lung adenocarcinoma cell line A549 (donated by the Cancer Institute of Yunnan Cancer Hospital), human transforming growth factor (PeproTech Company), PHA (Phytohemagglutinin) 2 mg/branch of plant location (SIGMA Company, USA), and human transforming growth factor (SIGMA Company, USA). Lymphocyte isolation solution (SIGMA Company, USA). Anti-CD3-FITC antibody (Anti-CD3-fluorescein isothiocyanate antibody) (serial number: A07746), anti-CD4-PE (CD4-Phycoerythrin) antibody (serial number: A07751), anti-CD8-PE antibody (serial number: A07753); Anti-CD25-PC5 antibody (no: IM2636), human Th1/Th2 cytokine kit (BD Company, USA). RPMI-1640 medium (Invitrogen, USA). Primary polystyrene disposable culture bottle measuring 15 mL/20 mL/150 mL; primary polypropylene one-time centrifuge tube 50 mL, primary polypropylene 6-well cell culture plate; primary polypropylene straight wall 50 mL cell culture bottle; Primary polypropylene straight wall 200 mL cell culture flask (Corning company, USA). Inverted phase contrast microscope (U-LH100HG, OLYMPUS Optical, Japan); Electron microscope (EVO LS10, Carl Zeiss, Germany); Flow cytometry (Epics XL, Beckman Coulter, USA).
Experimental methods
Culture of human lung adenocarcinoma cell line A549
A549 cells were cultured in RPMI1640 complete medium in a saturated humidity incubator under the conditions of 37 °C and 5% CO2. The complete medium held 10% fetal bovine serum. All the cells were subcultured once every 4 days, and the culture medium was replaced every 1 day. The cells were used in the experiment when the cells were in the logarithmic phase.
Isolation and purification of PBMCs from patients with NSCLC
Peripheral venous blood samples from patients with NSCLC (11 mL) were collected by aseptic vacuum heparin anticoagulation. 1 mL was used to detect the activity, purity, and number of T cells in peripheral mononuclear cells (PBMCs). First. PBMCs were separated according to the Ficoll stratified solution method. RPMI-1640 medium containing 10% fetal bovine serum was used to adjust the culture medium to 5 mL. PHA (10 µg/mL) was added to stimulate lymphocyte proliferation and transferred into a 20 mL cell culture flask, and the culture bottle was laid flat. Cultured in 37 °C, 5% CO2, saturated humidity incubator; The cell growth and morphological changes were observed by inverted phase contrast microscope two hours after PBMCs isolation and culture and 48 hours after adding PHA (10 µg/mL). Second. The peripheral venous blood (1 mL) oscillated on the oscillator for 30 seconds, and anti-CD3-FITC antibody, anti-CD4-PE antibody, anti-CD8-PE antibody, and anti-CD25-PC5 antibody was added to the flow cytometry. The excitation light was excited by 488nm on the argon laser. The specific surface antigen markers of T cells and the corresponding IgG staining cells were used as the negative control. The detection results were calculated according to the marker curves of each group. The harvest rate of PBMCs was calculated, and the mononuclear cells with purity >95% and good activation degree were selected for proliferation and culture.
A549 was co-cultured with PBMCs suspension and TGF-β1 factor, and the morphological changes were observed by light microscope
A549 cells in logarithmic growth phase were planted in a 6-well cell culture plate of original polypropylene with an adjustment of 1×106/mL for a total of 60 wells, and cultured in an incubator at 37 °C, 5% CO2 and saturated humidity. The cells were fused at 60–70%. The serum-containing medium was sucked up and washed with PBS for 3 times. The cells were cultured in 1 mL serum-free RPMI-1640 medium. The proliferated mononuclear cell suspension was adjusted to 1×106/mL after cell counting. Ten percent fetal bovine serum from 1 mLRPMI-1640 culture medium was added to the porous plate of A549 cell line with a sampling gun, and 1 mL was added to each plate. The seed plate holes were divided into a control group without TGF-β1-factor group with a total of 12 holes, experiment 2, 5, 10, 20 ng/mL groups, each group had 12 holes. A sampling gun was used to prepare TGF-β1 factor 6, 15, 30, and 60 ng/mL, respectively, to the pores of the experimental group. 10% of fetal bovine serum was added to the control medium. The amount of culture medium per well was adjusted to 3 mL, and the cells were cultured at 37 °C in 5% CO2 saturated humidity incubator. At the time point of mixed culture (Jeny3, Jeny5, Jeny8 d ) and inverted microscope fiber mirror (inverted phase contrast microscopezer, IPC), 10×4, 10×10, 10×40 times eyepiece were photographed, and then 100 cells with complete morphology in the visual field were randomly selected by Digimizer3.7 medical image analysis software after being photographed under 10×4, 10×10 and 10×40 eyepiece, respectively, at the time point of mixed culture (Jeny3, Jeny5, Jeny8 d ). The changes in cell number and morphology were observed and compared.
Flow cytometry observation of PBMCs cell membrane antigen at 3, 5 and 8 days after addition of TGF-β1 factor
As mentioned earlier, the centrifugally precipitated PBMCs cells in the supernatant were at once sent to fluorescence flow cytometry at 3, 5, and 8 days. The cells were suspended in 50 µL staining buffer, and the corresponding supernatant and cell surface staining reagent were added to each group: T cell surface-specific antigen Human Th1/Th2 cytokine Kit II monoclonal antibody was incubated at room temperature for 30 min, 1 mL PBS (phosphate buffer saline) was resuscitated and washed once. Absorb and discard the supernatant. The cells were resuscitated with 100 µL fixed permeability (cytofix/cytoperm) buffer. The monoclonal antibody was added and detected by flow cytometry. The excitation light was excited by 488 nm on the argon laser, the specific surface antigen marker of T cells, and the corresponding IgG staining cells were used as the negative control. The detection results were calculated according to the marker curves of each group. Prepare 3 rehearsals for each test and repeat 3 times.
Determination of Th1/Th2 factor level in the supernatant by flow cytometry
Mixed cell culture, as mentioned earlier, the supernatant 3 mL of each concentration of pore plate was centrifuged at 1,000 RPM/min for 10 minutes at 3, 5, and 8 days, and then the supernatant 2 mL was put into the aseptic EP tube for detection. 488nm excitation wavelength was used in flow cytometry. The prepared cells were detected by 525 nm emission wavelength. Draw the standard curve according to the instructions for the use of the kit. The OD value of each standard sample/specimen should be subtracted from the zero empty OD value. Take the standard sample concentration as the horizontal coordinate and OD value as the vertical coordinate to draw the standard curve and calculate the sample concentration to be measured. Prepare 3 tubes for each test, repeat 3 times.
Statistics
All the experiments were done in triplicate. All the data were statistically processed by SPSS18.0 statistical software. The immune parameters and cytokine concentrations of TGF-β1 (2, 5, 10, 20 ng/mL) and the group without TGF-β1 were evaluated for homogeneity of variance. If the variance was homogeneous, the one-way analysis of variance was used for the F test, and the test level α=0.05, P<0.05, P<0.01, the difference was statistically significant. There were differences among multiple groups, and then adjusted the test level to make a pairwise comparison, and analyzed the changes of cellular immunity and cytokines in TGF-β1 (2, 5, 10, 20 ng/mL) groups and the groups without TGF-β1 (2, 5, 10, 20 ng/mL).
Results
An inverted phase contrast microscope observed the changes in cell growth and morphology
Two hours after isolation, PBMCs began to show cluster-like aggregation, forming island-like suspension growth clusters, and a large number of mononuclear cells were diffusely suspended (see Figure 1A). Forty-eight hours later, the island-like growth, the mass increased significantly, the density became larger and showed suspension-like growth, and the isolated suspension growth of mononuclear cells was rare (see Figure 1B).
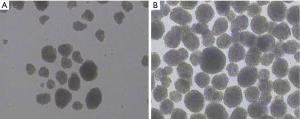
Observe the changes before and after adding TGF-β1 factor and PBMCs cells
After adding TGF-β1 and PBMCs cells, the morphology of A549 cells was elongated, the fusiform and the space between cells became larger, and the cells grew in loose connection between the cells, and the human lung adenocarcinoma cells were cobblestone-like, and the cells were in the form of tight junction, and the morphology of A549 cells was elongated, and the space between cells became larger. With the increase of TGF-β1 concentration and the prolongation of action time, the greater the difference of cell morphology (see Figure 2A,B,C,D,E,F,G,H,I,J).
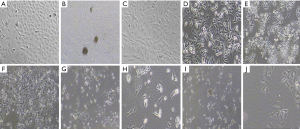
Effects of TGF-β1 on the immune function of PBMCs cells
The proportion of CD3 +, CD4 +, CD8 + and CD3 + CD25 + cells in PBMCs in TGF-β1 group was significantly lower than that in non TGF-β1 group (P<0.05). The proportion of CD4+CD25+ cells with TGF-β1 group was significantly higher than that in non TGF-β1 group (P<0.01). Within a certain range, the higher the concentration of TGF-β1, the longer the action time, the stronger the effect (Figure 3A,B,C,D,E,F).
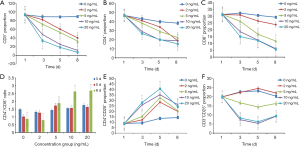
Effects of TGF-β1 on Th1 and Th2 factors in A549 cell line
Compared with the group without TGF-β1, the concentration of Th1 factor: IL-2, TNF, IFN-γ decreased in the TGF-β1 group, while the concentration of Th2 factor IL-4 and IL-10 increased (P<0.05). In a certain range, the higher the concentration of TGF-β1, the longer the action time, and the effect was enhanced. There was no significant correlation between the concentration of IL-6 and the concentration and action time of TGF-β1 compared with the group without TGF-β1 (P>0.05) (Figure 4A,B,C,D,E,F).

Discussion
Transform growth factor-beta1 (TGF-β1) is a complex cytokine (5), which widely exists in normal and cancerous tissues or cells in vivo. TGF-β1 is closely related to the tumor and plays a dual role in regulating the proliferation and differentiation of tumor cells (6). The study (7) showed that TGF-β1 was highly expressed in patients with NSCLC, which could promote the growth, invasion and metastasis of NSCLC. Comparable results have been obtained in vitro and in vivo in studies of colon cancer, liver cancer, gastric cancer, lung cancer and prostate cancer (8,9).
TGF-β1 is also an active tumor immunosuppressive factor, and its immunosuppressive effect is 104 to 106 times higher than that of cyclosporine A (Cyclospo rinse, CsA) (10). The metastasis ability of tumor cells depends on the loss of cell-to-cell junctions and the acquisition of fibroblasts characteristics, a process known as epithelial-interstitial transformation (11) (epithelial-mesenchymal transitions, EMT). In this study, the number of cell morphology EMT decreased and the cell membrane integrity was damaged at the same time, which contradicted other reports that TGF-β1 did not affect the proliferation of A549 cells, which may be related to the addition of PBMCs. In the tumor microenvironment, the apoptosis effect of CD4 + T cells on A549 cells is related.
Pirozzi et al. found that lung cancer cells were fusiform, and polarization changed in human lung cancer cell line A549 and human lung squamous cell carcinoma LC31 (lung cancer 31) treated with TGF-β1. Moreover, the expression of E-cadherin decreased, TGF-β also induced the nuclear production of β-catenin, and the change of EMT appeared (12), which enhanced the ability of migrant and local invasion of lung cancer cells.
Immunity in patients with lung cancer is an immune response dominated by cellular immunity (13). The main cells that play a key role in the immune mechanism are T lymphocytes, NK cells, and macrophages. Studies have shown that tumor patients with low cellular immune function, T lymphocytes, and NK cell activity decreased; at the same time, the malignant tumor produces a large number of immunosuppressive factor TDSF, which is one of the main causes of immune dysfunction in malignant tumor patients (14). Miki’s study shows that TGF-β1 has an extensive immunosuppressive effect (15), which may promote tumor growth by destroying immune surveillance and immune killing. Liu et al. (16) showed that there was a positive correlation between TGF-β1 and CD4+CD25+ cells, and the higher the proportion of CD4+CD25+ cells. TGF- beta 1, IL-10 and IL-2 are known to be involved in the transformation of CD4+CD25+ cells. An increase in CD4+CD25+ cells shows that tumor cells evade immune surveillance. Our study suggested that the proportion of CD4+CD25+ cells increased and then decreased compared with the group without TGF-β1. However, the proportion of CD4+CD25+ cells was still higher than that of the group without TGF-β1. With the increase of TGF-β1 concentration, the increased proportion of CD4+CD25+ cells increased (P<0.05).
The escape of lung cancer cells from immune surveillance is closely related to the occurrence, development, recurrence, metastasis, and prognosis of lung cancer. CD 4 cells are essential helper T lymphocytes (T help, Th cells). According to the cytokines secreted, they can be subdivided into two subsets: Th1 cells and Th2 cells. It is considered that the level of Th1/Th2 is closely related to the escape of lung cancer cells from immune surveillance. Th1 cells secrete cytokines such as IL-2, IL-12, IFN-γ and tumor necrosis factor TNF, which are mainly involved in cellular immune function (17) and mediate the immune response related to cytotoxicity and local inflammation. It is often regarded as hypersensitive T cells. Th2 cells secrete cytokines such as IL-4, IL-5, IL-6 and IL-10, which are involved in the regulation of immunosuppression. Th2 can also stimulate the proliferation of B cells to produce antibodies and take part in humoral immune function. The results showed that TGF-β1 (2, 5, 10, 20 ng/mL) group had an inhibitory effect on Th1 factor: IL-2, TNF, IFN-γ in the supernatant of A549 at 3, 5 and 8 d. It can promote Th2 factors: IL-4 and IL-10, and the higher the concentration of TGF-β1 is, the longer the action time is, and the effect increases.
There are few studies on the role of TGF-β1 in NSCLC, the mechanism of immune inhibition, the signal number pathway of the immune response, and the specific role of related cytokines. Therefore, the immunosuppressive effect of TGF-β1 on NSCLC has a broad prospect, which needs to be further studied and discussed.
Acknowledgments
Funding: National Natural Science Foundation of China (81960335); Natural Science Foundation of Yunnan Province (2017FA039); Natural Science Foundation of Yunnan Province (2017FE468-214); Medical Experts Training Project of Yunnan Province (D-201641, H-2018025).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4437
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4437). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee approved this study of the third affiliated Hospital of Kunming Medical University (No. KY2017056). All the subjects had informed consent of the trial and signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Frumento G, Piazza T, Di Carlo E, et al. Targeting tumor-related immunosuppression for cancer immunotherapy. Endocr Metab Immune Disord Drug Targets 2006;6:233-7. [Crossref] [PubMed]
- Fridlender ZG, Crisanti MC, Albelda SM. The Role of Tumor-Associated Macrophages and Other Innate Immune Cells in Metastatic Progression of Lung Cancer. In: Keshamouni V, Arenberg D, Kalemkerian G, editors. Lung Cancer Metastasis. New York: Springer, 2009:217-39.
- Flavell RA, Sanjabi S, Wrzesinski SH, et al. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 2010;10:554-67. [Crossref] [PubMed]
- Wang J, Chen J, Zhang K, et al. TGF-β1 regulates the invasive and metastatic potential of mucoepidermoid carcinoma cells. J Oral Pathol Med 2011;40:762-8. [Crossref] [PubMed]
- Paucarmayta A, Taitz H, Casablanca Y, et al. TGF-β signaling proteins and CYP24A1 may serve as surrogate markers for progesterone calcitriol treatment in ovarian and endometrial cancers of different histological types. Transl Cancer Res 2019;8:1423-37. [Crossref]
- Navab R, Strumpf D, Bandarchi B, et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci U S A 2011;108:7160-5. [Crossref] [PubMed]
- Hayashida T, Jinno H, Kitagawa Y, et al. Cooperation of cancer stem cell properties and epithelial-mesenchymal transition in the establishment of breast cancer metastasis. J Oncol 2011;2011:591427. [Crossref] [PubMed]
- Lo JF, Yu CC, Chiou SH, et al. The epithelial-mesenchymal transition mediator S100A4 maintains cancer-initiating cells in head and neck cancers. Cancer Res 2011;71:1912-23. [Crossref] [PubMed]
- Wang WY, Yan FH, Yao LY, et al. Effects of CsA and Pg LPS on proliferation and TGF-β1 production of cultured human gingival fibroblasts. Stomatology 2010-01-003.
- Navab R, Strumpf D, Bandarchi B, et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci U S A 2011;108:7160-5. [Crossref] [PubMed]
- Maitah MY, Ali S, Ahmad A, et al. Up-regulation of sonic hedgehog contributes to TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS One 2011;6:e16068. [Crossref] [PubMed]
- Pirozzi G, Tirino V, Camerlingo R, et al. Epithelial to mesenchymal transition by TGFβ-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One 2011;6:e21548. [Crossref] [PubMed]
- Conroy H, Galvin KC, Higgins SC, et al. Gene silencing of TGF-β1 enhances antitumor immunity induced with a dendritic cell vaccine by reducing tumor-associated regulatory T cells. Cancer Immunol Immunother 2012;61:425-31. [Crossref] [PubMed]
- Miki K, Tanaka H, Nagai Y, et al. Transforming growth factor beta1 alters calcium mobilizing properties and endogenous ATP release in A549 cells: possible implications for cell migration. J Pharmacol Sci 2010;113:387-94. [Crossref] [PubMed]
- Liu VC, Wong LY, Jang T, et al. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol 2007;178:2883-92. [Crossref] [PubMed]
- Dumitriu IE, Dunbar DR, Howie SE, et al. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 2009;182:2795-807. [Crossref] [PubMed]


