
Development and formulation of the classification criteria for osteoarthritis
Introduction
Osteoarthritis (OA) is a leading cause of pain and disability. This multifactorial joint disease is associated with biomechanical, genetic and other factors (1). The prevalence of OA in the elderly aged over 60 is about 10–15% (2). The annual treatment cost of OA is about 3,000 US dollars per person, and the direct and indirect costs account for about 0.25–0.5% of the countries’ GDP (3). OA thus contributes to a huge economic burden to families and the society. In recent years, with the diversification of OA treatment options, the classification of OA to help to select the most appropriate type of treatment has become an increasingly important topic. The existing classification criteria have neither been widely recognized nor used in clinical practice. On the one hand, the classification may lack the support of relevant research evidence, on the other hand, no standardized protocol for detailed steps of the development or clinical verification of classification criteria has yet been established.
To better guide the choice of a precise treatment of OA, The Committee of Rheumatological and Immunological Experts of the Cross-Straits Medicine Exchange Association (SMEA) launched the Categorization of Osteoarthritis CHecklist (COACH) project while developing the 2019 Chinese Guideline for Diagnosis and Treatment of Osteoarthritis (hereinafter referred to as “OA Guideline”) (4). The present article describes the development process of the COACH. We present the following article in accordance with the AGREE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4673).
Methods
The development of COACH consisted of four phases: (I) panel selection, (II) generation of the classification factors, (III) optimization of the classification factors, (IV) approval of the final criteria.
Panel selection
To gather opinions suggestion on the development of COACH, a multidisciplinary panel consisted of experts in rheumatology, orthopedics, rehabilitation, radiology, and evidence-based medicine from hospitals and scientific research institutions in different regions was convened.
Generation of the initial classification factors
Questionnaire survey
We conducted questionnaire included two main questions: (I) how to classify the OA patients? (II) how to choose treatment based on the classification results for different patients? we analyzed survey results to obtain classification factors by using the framework analysis (5,6).
Literature review
The objective of the literature review was to collate the supporting evidence for classification factors. We searched the MEDLINE, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), Epistemonikos, Cochrane Library and Wanfang database on November 2018 for Chinese or English articles about OA and initial classification factors. Search terms including: “分类标准”, “评估标准”, “临床分型”, “Classification criterion”, “Clinical classification”, “因素”, “暴露”, “预测”, “风险”, “risk”, “factor”, “incidence”, “systematic review”, “Meta”, “系统评价”, “系统综述” and “荟萃分析”. Systematic reviews, randomized controlled trials, cohort studies and case-control studies were systematically searched in order to include high to low quality of evidence from different study types. We included studies reporting any specific data. We summarized the findings of evidence link to classification factors for consensus meeting.
Optimization of the classification factors
A writing group drafted classification criteria. We invited COACH Panel members to participate in consensus meeting and to provide feedback. We reviewed the comments and optimized classification criteria through discussions.
Approval of the final criteria
The checklist was presented and discussed in a workshop at the Academic Annual Meeting of the 6th Cross-Strait Medical and Health Exchange Association Rheumatology and Immunology Committee. In this workshop, we asked the participants to give an overall impression of the checklist. The core group discussed the results and agreed on the final list of items.
Results
COACH Panel
Thirty-six individuals participated in COACH Panel at the conference in Beijing on March 9th, 2018. In total, 32 from 14 provincial administrative regions in China, 4 from overseas. Roles of the panelists were rheumatologist (80.6%; 29/36), orthopedist (13.9%; 5/36), methodologist (2.8%; 1/36) and rehabilitation physician (2.8%; 1/36).
Generation of the initial classification factors
The survey questionnaire results included 10 classification factors from framework analysis. We retrieved 2,872 articles for literature review, from which we included 16 systematic reviews and 20 cohort studies as supporting evidence.
Optimization of the classification factors
The COACH group held two face-to-face meetings with same group of experts, in Beijing on May 25th and in Guangzhou on December 15th, 2018, selected all classification factors and integrated 3 classification factors on the classification model.
Final criteria and supporting evidence
The main body of the classification criteria consists of six types of OA pathogenesis, eight types of medical findings (which can be grouped into two categories), and six types of the location. OA patients are divided into different types according to the pathogenesis (purple boxes), the supporting data next to the box showing the risk ratio of pathogenesis. Medical findings are shown in dark blue circles, and the percentage on the left represent the frequency of OA patients. Treatments are shown in green (basic treatment, the ordinal number is the order in OA Guideline), yellow (medication), orange (surgery) and black (treatment of primary disease) boxes. The location of the disease is represented by a hexagon. The connection between treatments, medical findings and location represents the choice of treatment that should be considered (Figure 1).
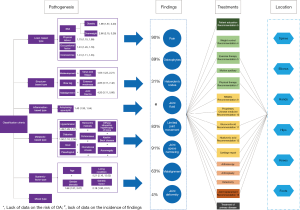
Load-based OA
Load-based OA develops mainly due to excessive joint load, occupational risks, exercise, overweight and obesity. This type of OA will exert repetitive stress, is often accompanied by the formation of osteophyte and subchondral cysts, and may result in destruction of articular cartilage. A systematic review from China published in 2017 showed an increased risk of knee OA in excessive joint-loaded populations [odds ratio (OR) =3.29, 95% confidence interval (CI): 1.76–6.15] (7). Another 2017 systematic review showed an increased risk of hip OA in senior athletes, especially handball, football and hockey players (8), and one systematic review showed that the risk of knee OA was increased in professional athletes (OR =1.31, 95% CI: 1.11–1.55) (9). Overweight and obesity can also cause greater load on joints, especially the weight-bearing joints, such as the hips and knees. A 2015 systematic review showed an increased risk of knee OA in overweight (OR =1.98, 95% CI: 1.57–2.20) and obese patients (OR =2.66, 95% CI: 2.15–3.28). About a quarter new cases of knee OA were caused by overweight or obesity (10).
Structure-based OA
Abnormal instability of joint structure, joint deformity or defect, and injury of bone, cartilage, ligament, meniscus and muscle weakness caused by cartilage movement and other factors, can lead to instability of joint structure and biomechanical changes of joint. The incidence of OA with hip dysplasia was found to be 80% (95% CI: 28–99%) (11). A systematic review showed that joint malalignment (12) and arched leg (13) are risk factors for OA. Patients with knee extensor muscle weakness had an increased risk of knee OA (OR =1.65, 95% CI: 1.23–2.21) (14). Knee varus and valgus increased the risk of medial compartment OA (OR =3.59, 95% CI: 2.62–4.92) and lateral compartment OA (OR =4.85, 95% CI: 3.17–7.42) (15). Joint trauma could also lead to abnormal joint structure. The risk of knee joint OA was significantly increased in patients with knee injury history (OR =4.20, 95% CI: 3.11–5.66) (16).
Inflammation-based OA
The inflammatory mediators, mainly caused by various types of inflammatory arthritis (suppurative, tuberculous, and rheumatoid arthritis), may affect synovial cells and result in synovitis and articular cartilage destruction. Inflammation at joint sites may cause OA (17). A cohort study from 2017 showed an increased risk of OA in patients with ankylosing spondylitis (OR =1.43, 95% CI: 1.33–1.54) (18).
Metabolic-based OA
Metabolic-based OA is mainly due to metabolic disorders and changes in the metabolic environment of the joints that lead to obstruction of bone formation, further destruction of articular cartilage and OA. We identified the following specific risk factors: (I) the inflammatory response and high glucose environment caused by diabetes metabolism aggravated the destruction of articular cartilage. A 2017 systematic review (19) showed an increased risk of OA in diabetic population (OR =1.50, 95% CI: 1.10–2.06). (II) There may be common risk factors between hypertension and OA, such as advanced age, obesity and chronic inflammation (20). A 2017 systematic review (20) showed that hypertensive population had an increased risk of OA (OR =1.49, 95% CI: 1.26–1.77). (III) Sodium urate crystals in the joints of gout patients bind to Toll-like receptors in chondrocytes, which affect chondrocyte function, and the pain caused by it may also alter OA biomechanics. A 2016 cross-sectional study (21) showed that gout patients had higher risk of onset of hand OA (OR =1.46, 95% CI: 0.61–3.50), having ≥8 hand joints with moderate to severe OA (OR =3.57, 95% CI: 0.62–20.45), foot OA (OR =2.16, 95% CI: 0.66–7.06), having ≥3 foot joints affected with OA (OR =4.00, 95% CI: 0.99–16.10), and having ≥1 foot joint with severe OA (OR =1.46, 95% CI: 0.54–3.94) than patients without gout. (IV) Calcium pyrophosphate dihydrate (CPPD), which often has similar symptoms than gout, is called pseudo-gout. The pathogenesis of CPPD is still unclear. Many cases of OA patients have CPPD. A case-control study from 1999 (22) showed that patients with CPPD (pseudogout) had an increased risk of OA (OR =13.8, 95% CI: 3.4–59.8). (V) The iron excess caused by hemochromatosis may lead to the inhibition of osteoblast activity and the reduction of bone formation. A 2010 case-control study (23) showed that people with hereditary hemochromatosis had an increased risk of OA (OR =2.5, 95% CI: 1.8–3.6), knee arthroplasty (OR =5.3, 95% CI: 1.1–25.6) and hip arthroplasty (OR =5.2, 95% CI: 2.2–11.9). (VI) Brown yellow disease leads to the accumulation of uric acid and the weakening of collagen, resulting in crevice and degeneration of articular cartilage, which is an important cause of OA joint replacement (24). (VII) Cartilage calcinosis may indirectly lead to cartilage degeneration by increasing matrix hardness. A 2013 cross-sectional study showed that patients with chondromatosis had an increased risk of right knee OA (OR =2.39, 95% CI: 1.79–3.20), right hip OA (OR =1.08, 95% CI: 0.73–1.59), right wrist OA (OR =4.46, 95% CI: 3.24–6.13), left knee OA (OR =2.78, 95% CI: 2.04–3.79), left hip OA (OR =0.72, 95% CI: 0.44–1.20), and left wrist OA (OR =4.42, 95% CI: 3.26–6.00) (25). (VIII) Kashin-Beck disease may increase the incidence of OA. An epidemiological study from 2018 showed that the detection rate of hand OA was 42.3%, and knee OA was 62.5% in patients with Kashin-Beck. In the patient group without Kashin-Back disease, the detection rate of hand OA was 33.3%, and knee OA was 56.6%. The difference was statistically significant (26). (IX) The risk factors of diffuse idiopathic bone hypertrophy (DIH) may also be related to OA. A 2015 population-based cohort study showed that the risk of OA was increased (OR =1.89, 95% CI: 1.14–3.10) (27) with DIH. (X) The risk of OA in patients with acromegaly was also significantly increased (28).
Systemic factor-based OA
This type of OA cannot be associated with any main pathogenic factor. Age is closely related to the occurrence of OA. The incidence of OA in people aged 40 years or above ranges between 10% and 17%, and among people aged 60 years and older the risk at least 50% (29). Potential reasons include the decline of age-related bone regeneration ability and the accumulation of risk factors (1). The risk of OA in women was significantly higher than that in men (OR =1.68, 95% CI: 1.37–2.07) (10), and sex hormones may be the cause of the difference. Decreased estrogen levels caused by ovarian dysfunction can lead to articular cartilage metabolism becoming weaker and inducing OA (30). A cold or humid living environment may also cause OA (31). A 2017 systematic review of the Chinese population showed an increased risk of knee OA among people living in humid environments (OR =5.21, 95% CI: 2.26–12.02) (7). The incidence of OA is also associated with certain genetic factors. Genome-wide association studies have shown that genetic polymorphism was associated with the susceptibility of OA. The British Association of Osteoarthritis Genetics has identified 11 OA-related genes in European populations (32). Two systematic reviews of Asian populations also showed that rs12885713 and rs7639618 gene polymorphisms were significantly associated with increased risk of OA (33,34).
Mixed type
This type is related to several of the factors mentioned above, without any factor being dominant.
Medical findings
The medical findings differ by type of OA. For example, because of subchondral bone hyperplasia, osteophytes and Heberden nodules are more easily detected by imaging examination in load-based OA; limited joint movement, joint deformity and abnormal joint alignment are more common in structure-based OA; and joint pain is more significant in inflammation-based OA. However, because this study has not carried out a survey of the incidence of findings of different types, only the retrieved data reported in the process of developing the classification criteria of the American Academy of Rheumatology (ACR) were presented.
Clinical findings
ACR classification criteria showed that the frequencies of OA pain in knee, hip and hand joints were 90%, 81% and 45%, respectively (35-37). The study of OA classification criteria for hip joint (36) showed that the frequency of limited joint movement was 83%.
Imaging findings
ACR classification criteria showed that the frequencies of osteophytes for hip and knee OA were 89% and 70%, respectively (36,37). The frequencies of joint fluid and joint space narrowing were 91% and 84% for hip and knee OA, respectively (36,37). One study of OA classification criteria for hand joints showed that the frequency of Heberden’s nodes was 31% (35). For knee joints, the frequency of malalignment was 63% (37). The frequency of joint deformity for hip OA was 4% (36).
Disease location
OA may occur in various synovial joints, including the hands, knees, hips, spine (e.g., cervical vertebrae, lumbar vertebrae), elbows, and feet. It may affect multiple joints in one person (38). The decision of treatment options, especially in surgical treatment, depends on the joints that it affects. Specific treatment options are presented in the OA guidelines.
Discussion
In this study, we constructed a framework for OA classification through systematic developing methods. The classification criteria combined classification factors of pathogenesis, findings and locations.
Pathogenesis was the most frequently mentioned and the most popular classification factor. The other were medical findings, treatments and location. After this, we built a preliminary classification model according to the relationship between the factors. There are differences in the frequency of medical findings in patients with different pathological causes. The treatment options should be chosen based on the medical findings. Part of the treatments are restricted to certain locations of OA.
The main future directions for the development of this classification criteria include: (I) exploring the pathological mechanism and frequency of OA, (II) studying the proportion of patients with different types of OA, (III) investigating the frequency of medical/clinical findings in patients with different types of OA, (IV) conducting clinical research to verify the application effect of classification criteria, and (V) further analyzing the relationship among treatments, findings and location.
Conclusions
This classification criteria can help clinicians quickly classify OA patients through the results of consultation, and they are also of great significance to early interventions for OA. Through the classification criteria and the OA guidelines, clinicians and patients can choose the best treatment option according to the types, findings and locations. In addition, the classification criteria are also helpful to promote the basic medical research and targeted prevention of OA.
Acknowledgments
We thank Nan Yang, Yanfang Ma, Jingyi Zhang, Jianjian Wang, Qi Zhou, Xufei Luo, Meng Lv and Zijun Wang of Lanzhou University for literature reviewing. We thank Janne Estill, Institute of Global Health of University of Geneva for providing guidance and comments for our classification criteria.
Funding: This study was supported by National Key R&D Program of China (2018YFC1705503).
Footnote
Reporting Checklist: The authors have completed the AGREE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4673
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4673
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4673). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet 2015;386:376-87. [PubMed]
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646-56. [PubMed]
- Puig-Junoy J, Ruiz Zamora A. Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Semin Arthritis Rheum 2015;44:531-41. [Crossref] [PubMed]
- Zhang Z, Huang C, Jiang Q, et al. Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition). Ann Transl Med 2020. [Crossref]
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. Journal of Administration and Governance 2009;4:72-9.
- Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG. editors. Analyzing qualitative data. London: Routledge, 1994:173-94.
- Ren Y, Shi Y, Tan B, et al. Meta-analysis of the risk factors for knee osteoarthritis among the Chinese population. Mod Prev Med 2015;42:2282-4.
- Vigdorchik JM, Nepple JJ, Eftekhary N, et al. What Is the Association of Elite Sporting Activities With the Development of Hip Osteoarthritis? Am J Sports Med 2017;45:961-4. [Crossref] [PubMed]
- Chen S, Wang X, Gao X. Risk Factors for Knee Osteoarthritis: a Systematic Review and Meta analysis. Geriatrics & Health Care 2016;22:405-10.
- Silverwood V, Blagojevic-Bucknall M, Jinks C, et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:507-15. [Crossref] [PubMed]
- Terjesen T. Residual hip dysplasia as a risk factor for osteoarthritis in 45 years follow-up of late-detected hip dislocation. J Child Orthop 2011;5:425-31. [Crossref] [PubMed]
- Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum 2009;61:459-67. [Crossref] [PubMed]
- Chapple CM, Nicholson H, Baxter GD, et al. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken) 2011;63:1115-25. [Crossref] [PubMed]
- Øiestad BE, Juhl CB, Eitzen I, et al. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:171-7. [Crossref] [PubMed]
- Sharma L, Chang AH, Jackson RD, et al. Varus Thrust and Incident and Progressive Knee Osteoarthritis. Arthritis Rheumatol 2017;69:2136-43. [Crossref] [PubMed]
- Muthuri SG, McWilliams DF, Doherty M, et al. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage 2011;19:1286-93. [Crossref] [PubMed]
- Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006;20:3-25. [Crossref] [PubMed]
- Lu MC, Tung CH, Yang CC, et al. Incident osteoarthritis and osteoarthritis-related joint replacement surgery in patients with ankylosing spondylitis: A secondary cohort analysis of a nationwide, population-based health claims database. PLoS One 2017;12:e0187594. [Crossref] [PubMed]
- Wang H, Chen J, Luo S, et al. Correlation between diabetes and osteoarthritis: a meta-analysis. Orthopedic Journal of China 2017;25:994-8.
- Zhang YM, Wang J, Liu XG. Association between hypertension and risk of knee osteoarthritis: A meta-analysis of observational studies. Medicine (Baltimore) 2017;96:e7584. [Crossref] [PubMed]
- Bevis M, Marshall M, Rathod T, et al. The association between gout and radiographic hand, knee and foot osteoarthritis: a cross-sectional study. BMC Musculoskelet Disord 2016;17:169. [Crossref] [PubMed]
- Stucki G, Hardegger D, Böhni U, et al. Degeneration of the scaphoid-trapezium joint: a useful finding to differentiate calcium pyrophosphate deposition disease from osteoarthritis. Clin Rheumatol 1999;18:232-7. [Crossref] [PubMed]
- Richette P, Ottaviani S, Vicaut E, et al. Musculoskeletal complications of hereditary hemochromatosis: a case-control study. J Rheumatol 2010;37:2145-50. [Crossref] [PubMed]
- Pierce TP, Issa K, Ramirez A, et al. Ochronosis as Etiology of Requiring Total Knee Arthroplasty-A Case Series. Surg Technol Int 2016;29:261-4. [PubMed]
- Abhishek A, Doherty S, Maciewicz R, et al. Evidence of a systemic predisposition to chondrocalcinosis and association between chondrocalcinosis and osteoarthritis at distant joints: a cross-sectional study. Arthritis Care Res (Hoboken) 2013;65:1052-8. [Crossref] [PubMed]
- Lian W, Liu H, Song Q, et al. Prevalence of hand osteoarthritis and knee osteoarthritis in Kashin-Beck disease endemic areas and non Kashin-Beck disease endemic areas: A status survey. PLoS One 2018;13:e0190505. [Crossref] [PubMed]
- Kagotani R, Yoshida M, Muraki S, et al. Prevalence of diffuse idiopathic skeletal hyperostosis (DISH) of the whole spine and its association with lumbar spondylosis and knee osteoarthritis: the ROAD study. J Bone Miner Metab 2015;33:221-9. [Crossref] [PubMed]
- Wassenaar MJ, Biermasz NR, van Duinen N, et al. High prevalence of arthropathy, according to the definitions of radiological and clinical osteoarthritis, in patients with long-term cure of acromegaly: a case-control study. Eur J Endocrinol 2009;160:357-65. [Crossref] [PubMed]
- Chinese Rheumatology Association. Guidelines for diagnosis and treatment of osteoarthritis. Chinese Journal of Rheumatology 2010;14:416-9.
- Hu S, Shen L. Plasma insulin-like growth factor-I level in the patients with diabetes mellitus. Chin J Endocrinol Metab 2001;17:162-3.
- Shen Y, Liu F, Cao H, et al. Clinical manifestation and influence factors in patients with knee osteoarthritis. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15:1643-6.
- Zeggini E, Panoutsopoulou K, Southam L, et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet 2012;380:815-23. [Crossref] [PubMed]
- Zhang R, Yao J, Xu P, et al. Association between genetic variants of DVWA and osteoarthritis of the knee and hip: a comprehensive meta-analysis. Int J Clin Exp Med 2015;8:9430-7. [PubMed]
- Zhang F, Wang X, Zhao P, et al. A comprehensive Meta-analysis of association between genetic variants of CALM1 and osteoarthritis. Chinese Journal of Clinicians 2015;9:4631-5. (Electronic Edition).
- Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990;33:1601-10. [Crossref] [PubMed]
- Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 1991;34:505-14. [Crossref] [PubMed]
- Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis Classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039-49. [Crossref] [PubMed]
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323-30. [Crossref] [PubMed]


