Diagnosis of Helicobacter pylori by invasive test: histology
Introduction
Half of the world’s population is infected with Helicobacter pylori (H. pylori) (1), and this gram-negative bacterium, which colonizes the gastric epithelium, is the major cause of gastric carcinogenesis and other gastric diseases, such as, chronic gastritis, gastroduodenal ulcers, and gastric mucosa-associated lymphoid tissue lymphoma (2). In fact, H. pylori was named a “definite biological carcinogen” by the World Health Organization in 1994 (3). The accurate detection of H. pylori is essential for managing infected patients and for eradicating the bacteria. Since the discovery of H. pylori, several diagnostic methods have been developed for the aim of accurate detection of this organism. These tests include noninvasive method—serology, urea breath test, or stool antigen test—and invasive methods, such as, culture, histological examination, and rapid urease test, which require upper gastrointestinal endoscopy to obtain gastric biopsy samples (4,5). Among these, histological examination is one of the most useful diagnostic tests for H. pylori infection (6). This article reviews the diagnosis of H. pylori by invasive testing that focuses on histology.
Histologic examination
Role of histologic staining
H. pylori, a spiral-shaped bacterium, can be seen in hematoxylin and eosin (H&E) staining and the sensitivity and specificity of H&E stain has been reported as 69-93% and 87-90%, respectively. However, the specificity can be improved 90-100% by using special stains such as modified Giemsa stain, Warthin-Starry silver stain, Genta stain, and immunohistochemical (IHC) stain (7,8).
H&E stain can directly identify H. pylori in a high-magnification field and evaluate the degree of inflammation (Figure 1A). However, when a low density of H. pylori and atrophic mucosal change are combined, it becomes difficult to see the organism. As Giemsa stain is easy to use, inexpensive, and provides consistent results; it is the preferred method in many laboratories (Figure 1B) (9,10). Warthin-Starry silver stain was crucial to the original demonstration of H. pylori, but it is expensive and the results are not always reliable (Figure 1C) (11). Genta stain has the advantage of visualizing both the inflammatory cells and H. pylori by combining silver, H&E, and Alcian blue stains (12). However, this is technically complex, expensive and time-consuming. IHC staining is also available and highly sensitive and reliable. IHC stain have a particular advantage in patients partially treated for H. pylori gastritis, a setting that can result in atypical (including coccoid) forms, which may mimic bacteria or cell debris on H&E preparations (Figure 1D). The major advantages of IHC stain include shorter screening time and high specificity because it can exclude other similar-shaped organisms (13).
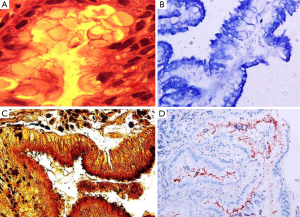
Laine et al. (7) compared these three stains (H&E, Giemsa, and Genta) and found the sensitivities were comparable at both low H. pylori density (H&E, 70%; Giemsa, 64%; Genta, 66%), and at high H. pylori density (H&E, 98%; Giemsa, 96%; Genta, 97%). Specificity was excellent (98-100%) for the Genta and Giemsa stains at both low and high H. pylori density and the H&E stain at a high density; however, specificity decreased (90%) in the H&E stain in low-density H. pylori. Ashton-Key et al. (14) also reported that IHC stain was a highly sensitive method for identifying H. pylori in gastric biopsy specimens—more sensitive than traditional stains (H&E, Giemsa, and Warthin-Starry silver) and considerably easier to interpret. However, although it would be ideal, it is not practical to perform H. pylori IHC staining on every gastric biopsy specimen.
In routine practice, if it is possible, at least two kinds of stain methods are recommended for diagnosis. H&E staining is usually adequate and Giemsa stain seems to have advantage over other stains because of its simplicity and consistency. Specialized IHC stains may be useful in followings: (I) no bacteria are visible on H&E or Giemsa staining, but there is evidence of inflammation on histology; (II) post-treatment biopsy specimens for mucosa-associated lymphoid tissue lymphoma to ensure eradication therapy has been successful; and (III) biopsy specimens in which coccoid forms or other organisms are not conclusively identifiable as H. pylori using routine stains (14).
Pros and cons of histologic method
Table 1 is a summary of previous reports regarding the advantages and disadvantages of each diagnostic method (2,15-17). The advantages of histology include its ability to document H. pylori infection and it also provided more information about the degree of inflammation and associated pathology, such as, atrophic gastritis (AG), intestinal metaplasia (IM), gastric cancer, or lymphoma (6). However, it has several limitations, including higher cost, longer turnaround time, and dependence on the skills of the operator (18). The density of H. pylori can vary at different sites, possibly leading to sampling error (19), and the sensitivity of histology may decrease in patients taking antisecretory therapy, such as, proton pump inhibitor (PPI).
Another limitation of histology is interobserver variability in assessment (6,20-25). Previously, a study evaluated the reliability of H. pylori identification on H&E-stained gastric biopsy specimens by 20 pathologists; the results showed very poor sensitivity (66%) and suboptimal specificity (88%) (20). Another reliability study reviewing Warthin-Starry silver-stained slides showed that the kappa value for intraobserver agreement was 0.65-0.88 and interobserver agreement was 0.39-0.82 (23). Other studies on the reproducibility of histological data for Giemsa- or Genta-stained slides have reached a similar conclusion (24,25). This may be due to the discrepancies in feature evaluation of H. pylori or the pathologist’s observations, because pathology results are based on subjective interpretation of different features and classification (26).
The optimal biopsy site
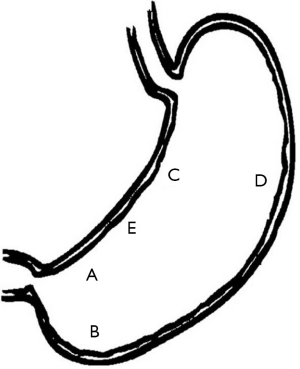
Despite high histology sensitivity, the site, number, and size of biopsy specimen affect diagnostic accuracy. Although a single biopsy taken from the angulus of the stomach, in untreated H. pylori positive patients, can detect H. pylori presence in more than 90% of cases (19), diagnostic accuracy can be increased with multiple biopsies from the greater curvature and the corpus. The updated Sydney system (27) recommends that biopsy specimens be taken at five different sites for optimal assessment of both gastritis and H. pylori status. In this system, each specimen should be obtained from the lesser and greater curvature of the antrum, both within 2-3 cm from the pylorus; the lesser curvature of the corpus about 4 cm proximal to the angulus; the middle portion of the greater curvature of the corpus, approximately 8 cm from the cardia; and from incisura angularis (Figure 2).
Histological test in special situation
Peptic ulcer bleeding
Peptic ulcer bleeding decreased the accuracy of H. pylori diagnostic test. A meta-analysis evaluating the histological sensitivity and specificity for the diagnosis of H. pylori infection in patients with upper gastrointestinal bleeding calculated a mean sensitivity of only 70% (28). However, in the presence of ulcer bleeding, histologic examination is the most reliable test. There have been studies indicating that histology was more sensitive than rapid urease test in diagnosing H. pylori infection in the peptic ulcer bleeding setting (29-31). Choi et al. (32) reported that peptic ulcer bleeding decreased the sensitivity of rapid urease test or culture, whereas, histology was found to be a quite reliable test, regardless of the presence of the bleeding. And serology showed relatively high sensitivity, but cannot be recommended as the first diagnostic method in bleeding situation, because of its low specificity. Choi et al. (32) also showed higher sensitivity than previous studies by using modified Giemsa stain rather than H&E stain and by obtaining many biopsy specimens.
Atrophic gastritis (AG) and intestinal metaplasia (IM)
When atrophic changes occur in the gastric mucosa, a high percentage of endoscopic biopsy samples become negative for a bacterial histology (33). Moreover, in metaplastic areas, H. pylori is undetectable by either conventional or special staining techniques in the majority of cases, despite serologic evidence of infection (34). The disappearance of H. pylori correlates with the development of IM and hypochlorhydria, which seem to be unfavorable environments for H. pylori colonization (35). In addition, the low prevalence of H. pylori in antral biopsy specimens of atrophic mucosa may be explained by a patchy distribution of the bacterial infection or a shift in colonization to the proximal stomach (corpus and fundus) resulting from hostile antral conditions including increasing pH where atrophy and IM occur more frequently (36,37).
After chronic H. pylori infection, AG and IM begin in the antrum and extend to the corpus along the lesser curvature side. As a result, lesser curvature side is not a good biopsy site for H. pylori detection because it is more susceptible to IM than is the greater curvature side. However, biopsy sampling from the lesser curvature of the corpus is known to be the most sensitive and appropriate for evaluation of gastric atrophy regression after H. pylori eradication therapy (38).
The effect of AG and IM on H. pylori detection rates is quite different between the Campylobacter-like organism test (CLO test) and Giemsa stain method, depending on the stomach area biopsied (39). That is, CLO test positivity was markedly reduced when AG and IM were severe, but Giemsa stain was less affected by AG and IM, especially by AG, which is a less hostile environment than IM. These findings may be due to the different characteristics of the two tests. The CLO test depends on urease activity, the strongest enzyme in H. pylori, which makes the acidic gastric mucosal environment to neutral pH for survival of H. pylori (40) by use of Ure I channel in the internal plasma membrane (41,42). As a result, in AG or IM, which are less acidic conditions, the bacteria do not need to activate urease, thus reducing test sensitivity. In contrast, Giemsa staining is dependent on the morphology of H. pylori, and therefore it detects H. pylori presence regardless of activity, increasing the sensitivity of this test versus the CLO test (43,44). In addition, the body’s acid secretions, where parietal cells exist, may also be a reason for the higher sensitivity of Giemsa staining in spite of the presence of IM.
Gastric cancer
AG and IM are considered premalignant lesions of gastric cancer. Thus, the appropriate biopsy site for detecting H. pylori infection in gastric cancer patients is similar to that of AG or IM patients. Kim et al. (45) reported that the antrum showed 55% sensitivity and corpus lesser curvature side showed 80% sensitivity in detecting H. pylori, whereas the corpus greater curvature side showed 95% sensitivity. Enomoto et al. (46) also reported that the H. pylori detection rate varied from 30% at the antrum lesser curvature to 100% at the corpus greater curvature in surgically resected gastric specimens. There results suggest that the adequate biopsy site for detecting H. pylori in gastric cancer patients is the corpus, especially the corpus greater curvature side.
PPI therapy
There may be reduced histology sensitivity and specificity in patients taking antisecretory drugs, such as H2-receptor antagonist (H2RA), PPIs, bismuth, and antibiotics. One study demonstrated that histological evaluation of an antrum biopsy had a sensitivity of 91% in 35 patients not taking an antisecretory drug, 91% in 34 patients on an H2RA, and 75% in 12 patients on a PPI. However, histology on biopsies taken from the corpus was more reliable, with a sensitivity of 83% in patients on a PPI, 91% in patients on an H2RA, and 94% in patients not taking an antisecretory drug (47,48). PPIs have strong antigastric secretion effects, in addition to being bacteriostatic against H. pylori. Thus, bacterial density may diminish, particularly in the antrum, but also in the body (18,49). For patients who receive PPI therapy, a better approach is to discontinue the PPI before endoscopic biopsy (50). The Maastricht IV/Florence Consensus Report for the management of H. pylori infection recommended that in patients treated with PPIs, if possible, the PPIs should be stopped 2 weeks before testing by histology, culture, rapid urease test, urea breath test, or stool antigen test (15). In addition, antibiotics also should be stopped 4 weeks before these tests (51). A false-negative result in histology is more controversial than other diagnostic methods because specialized pathologists have diagnosed H. pylori in the presence of surrogate features, such as, polymorphonuclear cells, even though the bacteria are absent (15). H2RA may also lead to false-negative results, but to a much lesser extent (52,53).
Possible complication: post biopsy bleeding
The American Society for Gastrointestinal Endoscopy recommended continual use of anticoagulation drugs, including warfarin, when patients undergo upper endoscopy with cold biopsy (54). There are no clinical trials demonstrating an increased incidence of bleeding in patients who underwent upper endoscopy with biopsy while taking aspirin or clopidogrel. Moreover, there is evidence that continuing therapeutic anticoagulation with warfarin during the periendoscopic period has a low risk of bleeding in such low-risk procedures (54). Although endoscopic cold biopsy is regarded as a low bleeding risk procedure, the incidence of delayed bleeding after cold biopsy varies among studies (55-62). Furthermore, the influence of anticoagulation agent, including dual or triple therapy, on post biopsy bleeding remains unclear. Yamashita et al. (60) recently reported that the incidence of major, delayed post biopsy bleeding was 0.06% and that post biopsy bleeding rate in an anticoagulation group was 0.4%; these are significantly higher than that of patients not on anticoagulations. Therefore, the possibility of post biopsy bleeding should be taken into consideration at all time, but especially in those taking anticoagulation drugs. Careful drug histology taking and sufficient explanation about the bleeding risk before and after biopsy is needed. In our clinical practice, we recommend discontinuation of anticoagulation drugs 5-7 days before an endoscopy in low-risk patients for cardiovascular or neurovascular disease. In high-risk patients, gastroenterologists had better consult to a cardiology or neurology specialist regarding discontinuation of anticoagulation.
Doctor must pay particular attention to patients with hematologic disease whose platelet function is markedly decreased. Patients with liver cirrhosis, chronic kidney disease, or other chronic diseases, such as, diabetes mellitus also should be carefully monitored. For patients with chronic kidney disease, it is safer to conduct a gastric biopsy after dialysis than before (63).
Conclusions
Histology is an excellent method for detecting H. pylori and provides additional information about gastric mucosa. H&E with or without Giemsa staining are usually adequate. The update Sydney system recommends gastric biopsies from five different sites; however, if this is not possible, the gastric body greater curvature may be a better site to detect current H. pylori infections, especially in the presence of peptic ulcer bleeding, AG and IM, or gastric cancer. PPIs can affect the result of histology and should be stopped 2 weeks before testing. Post biopsy bleeding may be increased in patients on anticoagulation therapy, so careful precautions should be taken.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- World gastroenterology organization global guideline: Helicobacter pylori in developing countries. J Dig Dis 2011;12:319-26. [PubMed]
- McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597-604. [PubMed]
- Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 1994;61:177-240. [PubMed]
- Basset C, Holton J, Ricci C, et al. Review article: diagnosis and treatment of Helicobacter: a 2002 updated review. Aliment Pharmacol Ther 2003;17:89-97. [PubMed]
- Rautelin H, Lehours P, Megraud F. Diagnosis of Helicobacter pylori infection. Helicobacter 2003;8:13-20. [PubMed]
- Aydin O, Egilmez R, Karabacak T, et al. Interobserver variation in histopathological assessment of Helicobacter pylori gastritis. World J Gastroenterol 2003;9:2232-5. [PubMed]
- Laine L, Lewin DN, Naritoku W, et al. Prospective comparison of H&E, Giemsa, and Genta stains for the diagnosis of Helicobacter pylori. Gastrointest Endosc 1997;45:463-7. [PubMed]
- Fallone CA, Loo VG, Lough J, et al. Hematoxylin and eosin staining of gastric tissue for the detection of Helicobacter pylori. Helicobacter 1997;2:32-5. [PubMed]
- Cha MS. Comparative analysis of histochemical stains about detection of H. pylori in gastric mucosa. Korean J Clin Lab Sci 2007;39:223-30.
- El-Zimaity HM, Segura AM, Genta RM, et al. Histologic assessment of Helicobacter pylori status after therapy: comparison of Giemsa, Diff-Quik, and Genta stains. Mod Pathol 1998;11:288-91. [PubMed]
- Doglioni C, Turrin M, Macri E, et al. HpSS: a new silver staining method for Helicobacter pylori. J Clin Pathol 1997;50:461-4. [PubMed]
- Genta RM, Robason GO, Graham DY. Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol 1994;25:221-6. [PubMed]
- Jonkers D, Stobberingh E, de Bruine A, et al. Evaluation of immunohistochemistry for the detection of Helicobacter pylori in gastric mucosal biopsies. J Infect 1997;35:149-54. [PubMed]
- Ashton-Key M, Diss TC, Isaacson PG. Detection of Helicobacter pylori in gastric biopsy and resection specimens. J Clin Pathol 1996;49:107-11. [PubMed]
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64. [PubMed]
- Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102:1808-25. [PubMed]
- Peura DA, Crowe SE. Helicobacter pylori. In: Feldman M, Friedman LS, Brandt LJ. eds. Sleisenger and Fordtran’s gastrointestinal and Liver disease, 9th ed. Philadelphia: Saunders, 2010:833-43.
- Dickey W, Kenny BD, McConnell JB. Effect of proton pump inhibitors on the detection of Helicobacter pylori in gastric biopsies. Aliment Pharmacol Ther 1996;10:289-93. [PubMed]
- Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc 1994;40:342-5. [PubMed]
- Molyneux AJ, Harris MD. Helicobacter pylori in gastric biopsies--should you trust the pathology report? J R Coll Physicians Lond 1993;27:119-20. [PubMed]
- Talebkhan Y, Mohammadi M, Rakhshani N, et al. Interobserver variations in histopathological assessment of gastric pathology. Pathology 2009;41:428-32. [PubMed]
- Tepes B, Ferlan-Marolt V, Jutersek A, et al. Interobserver agreement in the assessment of gastritis reversibility after Helicobacter pylori eradication. Histopathology 1999;34:124-33. [PubMed]
- Christensen AH, Gjorup T, Hilden J, et al. Observer homogeneity in the histologic diagnosis of Helicobacter pylori. Latent class analysis, kappa coefficient, and repeat frequency. Scand J Gastroenterol 1992;27:933-9. [PubMed]
- el-Zimaity HM, Graham DY, al-Assi MT, et al. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol 1996;27:35-41. [PubMed]
- Andrew A, Wyatt JI, Dixon MF. Observer variation in the assessment of chronic gastritis according to the Sydney system. Histopathology 1994;25:317-22. [PubMed]
- Aktepe OC, Ciftci IH, Safak B, et al. Five methods for detection of Helicobacter pylori in the Turkish population. World J Gastroenterol 2011;17:5172-6. [PubMed]
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-81. [PubMed]
- Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol 2006;101:848-63. [PubMed]
- Tu TC, Lee CL, Wu CH, et al. Comparison of invasive and noninvasive tests for detecting Helicobacter pylori infection in bleeding peptic ulcers. Gastrointest Endosc 1999;49:302-6. [PubMed]
- Archimandritis A, Tzivras M, Sougioultzis S, et al. Rapid urease test is less sensitive than histology in diagnosing Helicobacter pylori infection in patients with non-variceal upper gastrointestinal bleeding. J Gastroenterol Hepatol 2000;15:369-73. [PubMed]
- Griñó P, Pascual S, Such J, et al. Comparison of diagnostic methods for Helicobacter pylori infection in patients with upper gastrointestinal bleeding. Scand J Gastroenterol 2001;36:1254-8. [PubMed]
- Choi YJ, Kim N, Lim J, et al. Accuracy of diagnostic tests for Helicobacter pylori in patients with peptic ulcer bleeding. Helicobacter 2012;17:77-85. [PubMed]
- Kokkola A, Rautelin H, Puolakkainen P, et al. Diagnosis of Helicobacter pylori infection in patients with atrophic gastritis: comparison of histology, 13C-urea breath test, and serology. Scand J Gastroenterol 2000;35:138-41. [PubMed]
- Loffeld RJ, Stobberingh E, Flendrig JA, et al. Helicobacter pylori in gastric biopsy specimens. Comparison of culture, modified giemsa stain, and immunohistochemistry. A retrospective study. J Pathol 1991;165:69-73. [PubMed]
- Craanen ME, Blok P, Dekker W, et al. Subtypes of intestinal metaplasia and Helicobacter pylori. Gut 1992;33:597-600. [PubMed]
- Testoni PA, Colombo E, Cattani L, et al. Helicobacter pylori serology in chronic gastritis with antral atrophy and negative histology for Helicobacter-like organisms. J Clin Gastroenterol 1996;22:182-5. [PubMed]
- Kang HY, Kim N, Park YS, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci 2006;51:2310-5. [PubMed]
- Sugiyama T, Sakaki N, Kozawa H, et al. Sensitivity of biopsy site in evaluating regression of gastric atrophy after Helicobacter pylori eradication treatment. Aliment Pharmacol Ther 2002;16 Suppl 2:187-90. [PubMed]
- Yoo JY, Kim N, Park YS, et al. Detection rate of Helicobacter pylori against a background of atrophic gastritis and/or intestinal metaplasia. J Clin Gastroenterol 2007;41:751-5. [PubMed]
- Vassallo J, Hale R, Ahluwalia NK. CLO vs histology: optimal numbers and site of gastric biopsies to diagnose Helicobacter pylori. Eur J Gastroenterol Hepatol 2001;13:387-90. [PubMed]
- Weeks DL, Eskandari S, Scott DR, et al. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 2000;287:482-5. [PubMed]
- Strugatsky D, McNulty R, Munson K, et al. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature 2013;493:255-8. [PubMed]
- Versalovic J. Helicobacter pylori. Pathology and diagnostic strategies. Am J Clin Pathol 2003;119:403-12. [PubMed]
- Shin CM, Kim N, Lee HS, et al. Validation of diagnostic tests for Helicobacter pylori with regard to grade of atrophic gastritis and/or intestinal metaplasia. Helicobacter 2009;14:512-9. [PubMed]
- Kim CG, Choi IJ, Lee JY, et al. Biopsy site for detecting Helicobacter pylori infection in patients with gastric cancer. J Gastroenterol Hepatol 2009;24:469-74. [PubMed]
- Enomoto H, Watanabe H, Nishikura K, et al. Topographic distribution of Helicobacter pylori in the resected stomach. Eur J Gastroenterol Hepatol 1998;10:473-8. [PubMed]
- Kalantar J, Xia HH, Wyatt JM, et al. Determination of optimal biopsy sites for detection of H. pylori in patients treated or not treated with antibiotics and anti-secretory drugs. Gastroenterology 1997;112:A165.
- Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 1998;93:2330-8. [PubMed]
- Logan RP, Walker MM, Misiewicz JJ, et al. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut 1995;36:12-6. [PubMed]
- Cohen H, Laine L. Endoscopic methods for the diagnosis of Helicobacter pylori. Aliment Pharmacol Ther 1997;11 Suppl 1:3-9. [PubMed]
- Braden B. Diagnosis of Helicobacter pylori infection. BMJ 2012;344:e828. [PubMed]
- Gisbert JP, Pajares JM. 13C-urea breath test in the management of Helicobacterpylori infection. Dig Liver Dis 2005;37:899-906. [PubMed]
- Graham DY, Opekun AR, Jogi M, et al. False negative urea breath tests with H2-receptor antagonists: interactions between Helicobacter pylori density and pH. Helicobacter 2004;9:17-27. [PubMed]
- Anderson MA, Ben-Menachem T, Gan SI, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc 2009;70:1060-70. [PubMed]
- Shiffman ML, Farrel MT, Yee YS. Risk of bleeding after endoscopic biopsy or polypectomy in patients taking aspirin or other NSAIDS. Gastrointest Endosc 1994;40:458-62. [PubMed]
- Gerson LB, Michaels L, Ullah N, et al. Adverse events associated with anticoagulation therapy in the periendoscopic period. Gastrointest Endosc 2010;71:1211-7.e2.
- Whitson MJ, Dikman AE, von Althann C, et al. Is gastroduodenal biopsy safe in patients receiving aspirin and clopidogrel?: a prospective, randomized study involving 630 biopsies. J Clin Gastroenterol 2011;45:228-33. [PubMed]
- Ono S, Fujishiro M, Kanzaki H, et al. Conflicting clinical environment about the management of antithrombotic agents during the periendoscopic period in Japan. J Gastroenterol Hepatol 2011;26:1434-40. [PubMed]
- Repici A, Hassan C, Vitetta E, et al. Safety of cold polypectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy 2012;44:27-31. [PubMed]
- Yamashita K, Arimura Y, Fukuda K, et al. Major bleeding after endoscopic biopsy in relation to use of antithrombotics. Endoscopy 2014;46:538. [PubMed]
- Sarkis F, Abu Daya H, Sharara A, et al. Delayed overt gastrointestinal bleeding after cold endoscopic biopsy. Endoscopy 2013;45:75-author reply 76. [PubMed]
- Yao MD, von Rosenvinge EC, Groden C, et al. Multiple endoscopic biopsies in research subjects: safety results from a National Institutes of Health series. Gastrointest Endosc 2009;69:906-10. [PubMed]
- Lee HJ, Kim JI. The biopsy of upper gastrointestinal endoscopy. Korean J Helicobacter Upper Gastrointest Res 2012;12:166-70.