
Absent filling of the superficial middle cerebral vein is associated with reperfusion but not parenchymal hematoma in stroke patients undergoing thrombectomy: an observational study
Introduction
Hemorrhagic transformation (HT) is the most feared complication of reperfusion therapy after acute ischemic stroke. HT is classified into two major categories based on the European Cooperative Acute Stroke Study (ECASS) definition: hemorrhagic infarct (HI) and parenchymal hematoma (PH) (1). The latter type has been associated with poor outcomes in ischemic stroke patients, especially for those who received intra-arterial therapy (2,3). Thus, the detection of PH may be a useful tool to guide the management of acute stroke.
Recently, the cerebral venous system was found to play a conflicting role in the prediction of PH (4,5). The superficial middle cerebral vein (SMCV) is a large superficial cerebral vein which can be easily identified on imaging. We have previously found that the absent filling of the SMCV (referred to as SMCV−) was associated with brain edema expansion and poor functional outcomes in acute ischemic stroke, but was not associated with PH (6). We considered that the SMCV− on the ischemic side represented venous blood stagnation or even thrombosis within cerebral venules of the SMCV drainage area. Therefore, SMCV− patients were unlikely to suffer a PH because of a lack of cerebral blood perfusion within the ischemic area if arterial clots were not removed. However, the majority of enrolled patients had no large cerebral artery occlusions (LAO) and did not receive thrombectomy.
It has also been reported that abnormal venous outflow may hinder the reperfusion of ischemic brain tissue in LAO even after successful recanalization (7). At present, there is a lack of research clarifying the relationship between SMCV and PH after cerebral blood perfusion restoration through thrombectomy in patients with acute LAO. Therefore, in this study, we aimed to investigate the relationship between SMCV−, reperfusion, and PH in acute LAO patients.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1154).
Methods
Ethics statement
The study was approved by the institutional ethics committee (NO. KY2017019), and informed consent was obtained from all patients. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki (as revised in 2013).
Setting
We retrospectively reviewed our prospectively collected database for consecutive acute LAO patients who received computed tomography perfusion (CTP) scan at admission and thrombectomy within 6 hours after stroke onset from May 2018 to May 2019 in Zhejiang Provincial People’s Hospital.
Participants
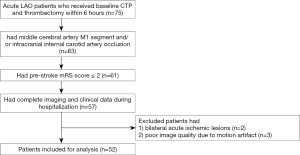
Patients were included if they had (I) a middle cerebral artery (MCA) segment 1 (M1) and/or intracranial internal carotid artery (ICA) occlusion on pre-operative 4D computed tomography angiography (4D-CTA) scans reconstructed from CTP; (II) a pre-stroke modified Rankin Scale (mRS) score ≤2; (III) complete imaging and clinical data during hospitalization. Patients were excluded if they had bilateral acute ischemic lesions or poor image quality due to motion artifacts (see Figure 1).
Reperfusion therapy
Patients who arrived at our center within 4.5 hours of symptom onset with no hemorrhage or significant low-density lesions on baseline non-contrast head CT (NCCT) images received alteplase at a dose of 0.9 mg/kg (maximum dose =90 mg). However, failure to receive thrombolysis did not preclude patients from thrombectomy. Patients with no clinical improvement after thrombolysis and patients who arrived 4.5–6 hours after stroke onset were thrombectomy candidates. According to the 2018 American Heart Association/American Stroke Association (AHA/ASA) guidelines, candidates were selected for thrombectomy if they met all of the following criteria (8): (I) pre-stroke mRS score of 0–1; (II) a causative occlusion of the ICA or MCA M1 segment; (III) aged ≥18 years; (IV) National Institutes of Health stroke scale (NIHSS) score of ≥6; (V) Alberta stroke program early CT score (ASPECTS) of ≥6; and (VI) treatment can be initiated (groin puncture) within 6 hours of symptom onset. Patients with any contraindication for thrombolysis who were eligible for thrombectomy also received thrombectomy directly.
Thrombectomy was performed as a bridging therapy after intravenous thrombolysis, or as primary therapy following the current guidelines. All patients who received thrombolysis or thrombectomy provided informed consent. The retrieval device for thrombectomy was the Solitaire AB (ev3, Covidian, Dublin, Ireland). The details of the surgical procedure performed are described in the EXTEND-IA trial (9). As the auxiliary treatment of thrombectomy, the use of tirofiban was initiated during and after thrombectomy. Patients received an initial dose of 0.4 mg over 30 minutes, and tirofiban administration was then continued at a dosage of 0.1 mg/kg body weight/hour. The recommended infusion duration was 24 hours.
Imaging protocols
All patients underwent baseline NCCT and CTP, and follow-up NCCT at 24–48 hours after admission. All CT imaging was acquired on a 320-detector row 640-slice cone-beam multidetector CT (MDCT) scanner (Aquilion One, Toshiba Medical Systems). Whole-brain NCCT was performed in one rotation (detector width 16 cm). After NCCT, a CTP was acquired after administration of 50 mL of contrast agent (Ultravist 370; Bayer HealthCare, Berlin, Germany) injected intravenously at a rate of 6 mL/second chased by 50 mL of saline (acquisition parameters: 120 kV, 128 mAs, scanning coverage =240 mm, scanning width =5 mm). Starting 7 seconds after contrast injection, a pulsed full rotation scan with 18-time points acquired over 60 seconds with a total pulse image acquisition time of 9.5 seconds was performed. The scanning protocol of NCCT performed 24 hours post-procedure was the same as that of baseline NCCT.
Imaging analysis
Two raters (S Zhang and Z Wang) who jointly evaluated the imaging characteristics were blinded to the patients’ follow-up imaging and clinical data. A single trained observer (S Zhang) measured imaging markers in all patients twice, at an interval of 1 month apart. Another observer (Z Wang) independently made the same evaluation.
Assessment of arterial collaterals
Multi-phase CTA, including arterial peak phase, venous peak phase, and late venous phase images, were generated according to the arterial input function/venous output function (AIF/VOF) curves by Vitoria® fX software (Version 1.0, Vital Images, Minnetonka, MN, USA). Leptomeningeal collaterals were assessed on multi-phase CTA using the method previously described in the ESCAPE trial (10). We divided patients into two groups: good and intermediate collaterals group, and poor collaterals group.
Absent filling of ipsilateral SMCV (SMCV−)
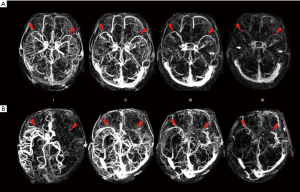
We assessed the SMCV on 4D-CTA reconstructed from CTP using Vitrea® fX (Version 1.0, Vital Images, Minnetonka, MN, USA). Images were analyzed using maximum intensity projection (MIP). SMCV was defined as negative (SMCV−) if no contrast filling of the SMCV across the whole venous phase in the ischemic hemisphere was present. The presence of contrast filling in the ipsilateral SMCV at any time point of the venous phase was classified as SMCV+ (refer to Figure 2). Patients with contralateral absent contrast filling of the SMCV only were also ascribed to the SMCV+ group.
Neurological outcome measures
NIHSS scores were evaluated and recorded at baseline, 24–72 hours after admission (as NIHSS within 24 hours was not acquired in some patients who received oral intubations or general anesthesia for thrombectomy), and at any time if a patient’s neurological status worsened.
Reperfusion and recanalization status were both assessed on digital subtraction angiograms (DSA) using modified thrombolysis in cerebral infarction (mTICI) scores (11) and arterial occlusive lesions (AOL) (12) respectively in patients who received thrombectomy. The mTICI and AOL scores were assigned following completion of the thrombectomy procedure. We defined an mTICI score of 0–2a as no reperfusion and a score of 2b–3 as reperfusion. AOL scores of 0–1 were defined as no recanalization, and scores of 2–3 were classified as recanalization.
HT was classified as HI or PH, according to the ECASS definition (1). Midline shift was defined as the displacement of the septum pellucidum (or cavum septi pellucidi) more than 3 mm from the spatial midline. HT and midline shift (13) were both assessed on the 24–48-hour NCCT scans.
mRS scores were used to identify the clinical outcome at discharge. mRS scores of 0–3 were classified as a good outcome, and mRS scores of 4–6 were classified as a poor outcome.
Statistical analysis
Cohen’s kappa coefficient was used to assess the level of inter- and intra-observer agreement for detecting the presence of SMCV−, poor collaterals, midline shift, and PH. Excellent inter- and intra-observer agreement was seen in distinguishing the SMCV− (κ=0.918 and 0.879), poor collaterals (κ=0.912 and 0.824), midline shift (κ=0.906 and 0.803) and PH (κ=0.924 and 0.954).
All numeric variables were expressed as mean ± standard deviation (SD) and median (interquartile range, IQR). Categorical variables were presented as frequency (percentage). Fisher’s exact test was used to compare dichotomous variables between groups, while the Mann-Whitney U test was used for ordered categorical variables. An independent-samples two-tailed t-test or Mann-Whitney U test was used for continuous variables, depending on the normality of the distribution. Variables identified by univariate analysis (P<0.1) were included in the binary logistic regression model. All analyses were performed with blinding of the participants’ identifying information. Statistical analysis was performed using SPSS 18 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
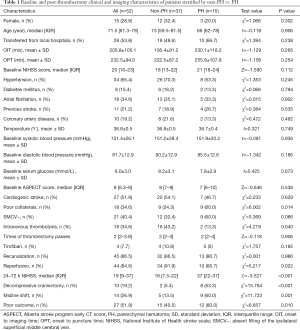
A total of 52 patients were enrolled in the study (Figure 1). Patients had a median age of 71.5 years (IQR, 61.3–79 years), and 15 (28.8%) were females. The median baseline NIHSS score was 20 (IQR, 16–23). SMCV− was found in 21 patients (40.4%) (univariate comparisons between SMCV− and SMCV+ are shown in Table S1). A total of 18 patients (34.6%) underwent bridging therapy, and 34 (65.4%) underwent thrombectomy alone. After thrombectomy, 44 patients had successful reperfusion (84.6%), and 15 (28.8%) had PH within 48 hours after thrombectomy. In patients who suffered PH, 60% had a midline shift, and 80% had poor outcomes. Baseline and post-thrombectomy clinical and radiological characteristics of all 52 patients are described in Table 1.
Full table
The association between PH and SMCV−
Before thrombectomy, compared with the non-PH group (n=37), the PH group (n=15) had a higher rate of poor collaterals and a lower rate of intravenous thrombolysis. The presence of SMCV− was not significantly different between the non-PH and PH group (Table 1). We enrolled in the SMCV− group, poor collaterals group, and the intravenous thrombolysis group into a binary logistic regression analysis and found that only intravenous thrombolysis was independently associated with PH [odds ratio (OR) =0.176, 95% confidence interval (CI): 0.031–0.996, P=0.049]. After thrombectomy, patients with PH had a lower rate of reperfusion, a higher 24-hour follow-up NIHSS score, a higher rate of midline shift, decompressive craniectomy, and poor outcomes at discharge (Table 1). We further added reperfusion into the binary logistic regression analysis and found that intravenous thrombolysis (OR =0.104, 95% CI: 0.013–0.831, P=0.033) and reperfusion (OR =0.110, 95% CI: 0.013–0.913, P=0.041) were both independently associated with PH, while SMCV− was still not associated with PH (OR =0.722, 95% CI: 0.102–5.090, P=0.744) (see Table S2).
The relationship between SMCV−, reperfusion, and PH
There was no significant difference in the rate of SMCV− between the recanalization and no recanalization group (37.8% vs. 57.1%, χ2=0.944, P=0.331). A significantly lower rate of SMCV− was found in the reperfusion group compared with the no reperfusion group (34.1% vs. 75.0%, χ2=4.705, P=0.030) (Table S3). Binary logistic regression analysis showed that SMCV− was an independent risk factor for reperfusion (OR =0.172, 95% CI: 0.031–0.960, P=0.045).
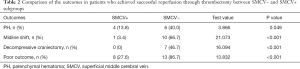
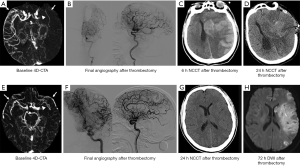
In the reperfusion group, patients with SMCV− had a higher rate of PH than patients with SMCV+ (40.0% vs. 13.8%, χ2=3.866, P=0.049) (see Table 2). In patients with SMCV−, the rates of PH were similar between the no reperfusion and reperfusion group (50% vs. 40.0%, χ2=0.175, P=0.676). In patients with SMCV+, the rate of PH was significantly lower in the reperfusion group compared to the no reperfusion group (13.8% vs. 100%, χ2=8.908, P=0.032). Examples of the relationship between SMCV−, reperfusion, and PH are shown in Figure 3.
Full table
Discussion
In this study, we found that SMCV− was a risk factor for reperfusion, but not for PH. Furthermore, in patients who achieved successful reperfusion, SMCV− had a higher rate of PH than SMCV+. The occurrence of PH can be influenced by intravenous thrombolysis before thrombectomy and reperfusion through thrombectomy.
In patients who received thrombectomy, the presence of SMCV− did not interfere with the rate of recanalization, meaning that the abnormality of SMCV drainage has no impact on recanalization since this mainly depends on the stent device or the characteristics of the clots. However, we found that SMCV− patients were unlikely to achieve successful reperfusion after thrombectomy. This result indicated that SMCV− at baseline might be involved in the hindrance of peripheral reperfusion even after arterial occlusion was resolved. This finding is consistent with the “no-reflow” phenomenon described by Gerber et al., where although the arterial opacification is normal, there is still a lack of contrast flow to the venous side after endovascular therapy (7). The occurrence of this phenomenon is most likely associated with “cerebral venous steal”. After a stroke, ischemia may damage small veins and venules, causing blood clot formation in the venous system. In this case, severe ischemia may lead to little outflow pass through the venous side, and the restored arterial blood flow by successful recanalization will diverge to the penumbra rather than to the core (the most ischemic area), thus “no-reflow” emerges (14,15).
In this study, reperfusion was found to be protective for the occurrence of PH, while SMCV− patients had a higher rate of PH than SMCV+ patients after reperfusion was successfully achieved (40% vs. 13.8%). Even after successful reperfusion from thrombectomy, the occurrence of PH was still high in SMCV− patients with the recovery of tissue pressure. Two mechanisms possibly explain this phenomenon. Firstly, the arterioles dilate 5–10 times the original lumen size because of the release of adenosine and nitric oxide from dying cells and the endothelium after brain ischemia (16). Zhang et al. also found that during cerebral ischemia, the arterial smooth muscle in downstream arteries from the clots may lose contractile ability to adjust blood flow in line with heart rate and blood pressure (17). In this case, when accompanied by drainage abnormalities of cerebral veins, we speculated that more blood might enter into the brain parenchyma resulting in reperfusion injury if the arterial clots were being removed by a stent retriever or a recombinant tissue plasminogen activator (rt-PA). Secondly, venules were also found to modulate the autoregulation of cerebral blood flow. Venules can have a protective effect by constricting pial veins to prevent the abrupt increase in venous pressure (18). However, in the acute phase after stroke, this protective effect could fail. When reperfusion happens, capillary and venules suffer a sudden elevation of perfusion pressure leading to regional hyperemia and disturbance of the venous brain-blood-barrier (19,20). Although it is the venule side that is reported to suffer HT due to brain-blood-barrier abruption and increased perfusion, it is not possible to evaluate the bleeding site in our population, which requires advanced imaging techniques in future studies.
Notably, we found that patients who received bridging therapy had a significantly lower rate of PH. The outcomes of direct thrombectomy vs. bridging therapy for acute LAO patients have long been debated. Maingard et al. reported that patients who received bridging therapy had a higher rate of reperfusion and functional independence (mRS 0–2 at 90 days) (21). Contrastingly, a network meta-analysis of 12 studies with a total of 3,161 patients showed that there was no significant difference in good functional outcome at 90 days between direct thrombectomy and bridging therapy (22). However, a comparison of the occurrence of PH between these two treatment options was rarely mentioned. In our study, intravenous thrombolysis might have induced clot lysis before thrombectomy or partially lysed the clot to permit some reperfusion before thrombectomy was conducted, resulting in a reduced time of cerebral hypoxia, mitigating irreversible damage (21,23).
As an imaging marker, SMCV− is recognizable, and the identification of SMCV− can be easily completed in the majority of CTP post-processing workstations with excellent inter- and intra-rater agreement. Therefore, its evaluation can be performed routinely before treatment decision-making in centers that have CTP scans. Although thrombectomy is now the primary treatment option for acute LAO, our study suggests that patients with SMCV− may face failure in reperfusion, and a higher risk of hematoma even if reperfusion can be successfully achieved. Therefore, a novel neuroprotective strategy for patients with SMCV− at baseline should be explored in order to provide feasible options in addition to thrombectomy.
This study had several limitations. Firstly, it had a small sample size and was a retrospective study, which created a potential risk of selection bias. Additionally, our subjects might be more severe than other centers in terms of neurological deficits (median NIHSS of 19). Therefore, results should be interpreted with caution. Secondly, as many patients did not receive further CTP, we cannot dynamically observe if the presence of SMCV− is influenced by reperfusion. Thirdly, the off-label use of tirofiban might have compromised study group homogeneity. Fourthly, we did not provide long-term follow-up data as we focused on observing the short-term changes in the clinical manifestation of acute LAO patients. In acute LAO, patients may suffer rapid deterioration of neurological function, which may even lead to death within several days after stroke onset. Therefore, short-term outcomes may be more reflective of prognosis than 3-month long-term outcomes in acute LAO patients. Our future studies will include both short- and long-term outcomes in order to comprehensively judge the predictive value of SMCV− in LAO patients receiving thrombectomy. Lastly, we did not evaluate the effect of other imaging markers on reperfusion after treatment, such as the length of the thrombus. Therefore, further studies are required using a panel of imaging markers to confirm our findings. Future investigations should also have a larger sample size and involve multicenter clinical trials to improve the generalizability of results.
In conclusion, in patients who received thrombectomy, SMCV− did not directly predict PH, but was a risk factor for reperfusion. Although reperfusion had a protective effect on PH, SMCV− patients still had a higher rate of PH than SMCV+ in patients who achieved successful reperfusion.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China [81801162 to SZ, 81702462 to CG.L.], National Natural Science the China Postdoctoral Science Foundation [2019M662083 to RT.Z.], Zhejiang Provincial Natural Science Foundation of China [LQ20H180015 to RT.Z.], and General Project Funds from the Health Department of Zhejiang Province [2013KYB024 to ZW].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1154
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1154
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-1154
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1154). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional ethics committee (NO. KY2017019), and informed consent was obtained from all patients. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Larrue V, von Kummer RR, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438-41. [Crossref] [PubMed]
- van Kranendonk KR, Treuniet KM, Boers AMM, et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg 2019;11:464. [Crossref] [PubMed]
- Lee JS, Hong JM, Kim EJ, et al. Comparison of the Incidence of Parenchymal Hematoma and Poor Outcome in Patients with Carotid Terminus Occlusion Treated with Intra-Arterial Urokinase Alone or with Combined IV rtPA and Intra-Arterial Urokinase. AJNR Am J Neuroradiol 2012;33:175-9. [Crossref] [PubMed]
- Parthasarathy R, Mahesh K, Rempel JL, et al. Prognostic evaluation based on cortical vein score difference in stroke. Stroke 2013;44:2748-54. [Crossref] [PubMed]
- Cartmell SCD, Ball RL, Kaimal R, et al. Early Cerebral Vein After Endovascular Ischemic Stroke Treatment Predicts Symptomatic Reperfusion Hemorrhage. Stroke 2018;49:1741-6. [Crossref] [PubMed]
- Zhang S, Lai Y, Ding X, et al. Absent Filling of Ipsilateral Superficial Middle Cerebral Vein Is Associated with Poor Outcome After Reperfusion Therapy. Stroke 2017;48:907-14. [Crossref] [PubMed]
- Gerber JC, Miaux YJ, von Kummer R. Scoring flow restoration in cerebral angiograms after endovascular revascularization in acute ischemic stroke patients. Neuroradiology 2015;57:227-40. [Crossref] [PubMed]
- Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2018;49:e46-e110. [Crossref] [PubMed]
- Campbell BCV, Mitchell PJ, Yan B, et al. A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy (EXTEND-IA). Int J Stroke 2014;9:126-32. [Crossref] [PubMed]
- Menon BK, d'Esterre CD, Qazi EM, et al. Multiphase ct angiography: A new tool for the imaging triage of patients with acute ischemic stroke. Radiology 2015;275:510-20. [Crossref] [PubMed]
- Dargazanli C, Consoli A, Barral M, et al. Impact of modified tici 3 versus modified tici 2b reperfusion score to predict good outcome following endovascular therapy. AJNR Am J Neuroradiol 2017;38:90-6. [Crossref] [PubMed]
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. [Crossref] [PubMed]
- Gibson JY, Massingale TW, Graves GR, et al. Relationship of Cranial Midline Shift to Outcome of Very-LowBirth-Weight Infants with Periventricular Hemorrhagic Infarction. J Neuroimaging 1994;4:212-7. [Crossref] [PubMed]
- Pranevicius M, Pranevicius O. Cerebral venous steal: blood flow diversion with increased tissue pressure. Neurosurgery 2002;51:1267-73. [Crossref] [PubMed]
- Pranevicius O, Pranevicius M, Pranevicius H, Liebeskind DS. Transition to collateral flow after arterial occlusion predispose to cerebral venous steal. Stroke 2012;43:575-9. [Crossref] [PubMed]
- Kulik T, Kusano Y, Aronhime S, et al. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology 2008;55:281-8. [Crossref] [PubMed]
- Zhang JH, Badaut J, Tang J, et al. The vascular neural network—a new paradigm in stroke pathophysiology. Nat Rev Neurol 2012;8:711-6. [Crossref] [PubMed]
- Fernández-Klett F, Priller J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J Cereb Blood Flow Metab 2015;35:883-7. [Crossref] [PubMed]
- Budohoski KP, Czosnyka M, Kirkpatrick PJ, et al. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol 2013;9:152-63. [Crossref] [PubMed]
- Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Transl Stroke Res 2016;7:294-302. [Crossref] [PubMed]
- Maingard J, Shvarts Y, Motyer R, et al. Outcomes of endovascular thrombectomy with and without bridging thrombolysis for acute large vessel occlusion ischaemic stroke. Intern Med J 2019;49:345-51. [Crossref] [PubMed]
- Phan K, Dmytriw AA, Lloyd D, et al. Direct endovascular thrombectomy and bridging strategies for acute ischemic stroke: a network meta-analysis. J Neurointerv Surg 2019;11:443-9. [Crossref] [PubMed]
- Barreto AD. Intravenous thrombolytics for ischemic stroke. Neurotherapeutics 2011;8:388-99. [Crossref] [PubMed]


