
A preliminary study on the visual outcomes after LaserACE for presbyopia
Introduction
Presbyopia is a ubiquitous visual disability of the aging eye. The presbyopic demographic is large and growing, with a high level of need for spectacle independence. In the last few decades, a number of surgical approaches for presbyopia correction have been used, including corneal refractive surgery, intraocular lenses implantation, and scleral surgery. Each approach has its own benefits and limitations (1-6). Accommodative approaches attempt to restore the true dynamic range of the defocusing ability of the eye. In contrast, pseudo-accommodative approaches provide functional near vision from a variety of non-accommodative factors.
Traditionally, presbyopia has been defined as the gradual loss of accommodation resulting from the loss of elasticity of the lens capsule and lens substance. Recent studies demonstrated that the ciliary body, zonules, anterior vitreous membrane, peripheral choroid as well as ocular rigidity may also play a role in presbyopia (7-10). Scleral surgeries were originally developed to meet the needs of restoring functional biomechanics in the accommodation apparatus. In this regard, the Laser Anterior Ciliary Excision (LaserACE, AceVision Group, USA) and VisAbility Implant System surgery (Refocus Group, Dallas, Texas, USA) have recently been under development (11-14). Lin began to utilize the erbium-YAG laser as a treatment for presbyopia, while it did not get developing (15). The LaserACE procedure is a scleral laser micro-excision procedure currently available designed to improve biomechanical mobility and compliance of scleral tissue, which utilizes the Visiolite erbium-YAG laser to ablate 600 µm laser spots in the sclera (11,12). A more compliant sclera may allow increased movement of the anterior zonule and greater accommodation. A clinical report has shown promising results for improving visual performance for near and intermediate visual tasks (12,13). In comparison with refractive approaches, scleral approaches could improve the near visual function without inducing optical changes or decreasing contrast sensitivity (16-18). In the current study, we reported the results of the comprehensive clinical outcomes after the LaserACE procedure for emmetropic patients over one year. To our knowledge, this study, for the first time, reported the result of the LaserACE procedure in the treatment of presbyopia in Mainland China.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2141).
Methods
Patients
This prospective, non-randomized, non-comparative study comprised four female patients with a mean age of 58.5±6.0 years (range, 52 to 66 years) at the Eye and ENT Hospital of Fudan University between April 2014 to August 2016. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Eye and ENT Hospital of Fudan University (No. ky2012-018) and informed consent was taken from all the patients after a complete description of the study. Inclusion criteria were at least 50 years of age, mean refractive spherical equivalent refraction (MRSE) of ±0.50 D for distance vision with astigmatism ≤1.00 D in each eye, uncorrected distance visual acuity (UDVA) of 20/40 (logMAR 0.30) or better in each eye, corrected distance visual acuity (CDVA) of 20/25 (logMAR 0.10) or better in each eye, and presbyopia ≥1.50 D at 40 cm. Exclusion criteria were a history of ocular trauma or prior ocular surgery except for corneal refractive surgery, ocular pathology other than cataract, history of nuclear sclerosis LOCS III grade 2 or worse or other cataracts reducing CDVA, and acute or chronic disease or illness that could increase the operative risk or confound the study outcomes.
Preoperative assessment
Clinical evaluations included visual acuity questionnaire assessments with Near Activity Visual Questionnaire (19) (Table 1); distance corrected near visual acuity (DCNVA) assessments with the logarithmic visual acuity chart-early treatment diabetic retinopathy study (ETDRS) 2000 Series at 40 cm, distance corrected intermediate visual acuity (DCIVA) assessments with the logarithmic visual acuity chart-ETDRS 2000 Series at 60 cm, UDVA and CDVA assessments with the logarithmic visual acuity chart-ETDRS 2000 Series at 4 m, manifest refraction, and near addition at 40 cm. The iTrace wavefront analyzer (Tracey Technologies, Houston, TX) was used to measure wavefront aberrations. The total higher-order aberrations (HOA), and spherical aberration (Z40) were calculated for the central 6-mm zone. The root mean square (RMS) was used to describe the HOA value. Pupil size was measured under photopic and mesopic lighting conditions with the NPi-200 pupillometer (Neuroptics, CA, USA). Slit lamp, intraocular pressure (IOP), and funduscopic examinations were also performed.

Full table
Surgical procedure
The LaserACE procedure was performed bilaterally for all subjects. All surgeries were performed by the same surgeon (XZ). The patients received the following medications 30 minutes prior to surgery: topical tobramycin, dexamethasone, and tetracaine. The patients received 1 drop of brimonidine 0.15% every 10 minutes for 3 doses over 30 minutes before surgery. Viscoelastic material (surgical gel) was placed over the cornea for protection. A corneal shield was placed on this gel over the cornea to block that area from any treatment during the procedure.
The LaserACE procedure was performed using the VisioLite erbium-YAG laser (ACE Vision Group, USA) at a specific wavelength of 2.94 µm with a laser frequency of 10–30 Hz, and spot size of 600 µm (12). Ablations were made in the sclera in three critical anatomic zones: 0.5 mm distance from the anatomical limbus to 1.1 mm over the region of the scleral spur and insertion of the ciliary muscle; 1.1 mm distance from the anatomical limbus to 4.9 mm over the center belly of the ciliary muscle and the thickest circumference of the sclera; and 4.9 mm distance from the anatomical limbus to 5.5 mm posterior to the pars plana, and anterior to the ora serrata over the origin of the ciliary muscle. Nine excisions were placed in the diamond matrix pattern in four oblique quadrants for a total of 36 neopores. The depth of the neopores was about 85–90% depth of the sclera, to the point that the blue hue of the choroid just became visible.
After the laser procedure, a collagen matrix powder mixed with a ratio of 1:4 (v/v) sterile saline solution in a 10 mL syringe was applied directly over the scleral ablation sites with a cannula. A scleral contact lens was routinely used postoperatively to cover the ablation zones. Patients received standard postoperative care and used antibiotic eye drops and dexamethasone four times a day for one week.
Postoperative follow-up examinations were scheduled for 1 day, 3 days, 1 week, 1, 3, and 6 months, and 1 year postoperatively. Comprehensive examinations were performed by the authors.
Statistical analysis
The statistical analyses were performed using the Statistical Package for Social Sciences software (SPSS, Version 22; IBM, Armonk, NY, USA). Data were expressed as mean ± standard deviation (SD). Repeated measures analysis was performed. In all tests, P<0.05 was deemed to indicate statistical significance.
Results
Patient characteristics
All four patients enrolled in this study completed each scheduled follow-up examination through 1 year. Table 2 shows the patients’ demographics and preoperative data.

Full table
Slit lamp microscopy and intraocular pressure
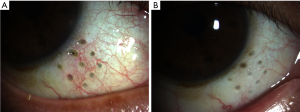
Mild conjunctival injection and edema were noticed in all eyes on 1 day postoperatively and relieved 1 week after surgery. The subconjunctival hemorrhage was observed in one eye (Patient 1) and it recovered after 1 week. At the 1-year visit, the anterior segment examination showed visible pores in the sclera covered by conjunctiva in four oblique quadrants. No evidence of scleral erosions was present (Figure 1).
The average IOP was 12.54±3.21 mmHg preoperatively, and 11.35±3.04 mmHg at 6 months, 11.89±2.98 mmHg at 12 months (P>0.05).
Near and intermediate visual acuity
Preoperative and postoperative monocular and binocular DCNVA and DCIVA are shown in Table 2. All four patients displayed improved binocular DCNVA and DCIVA. Three patients (3/4) had improvement in monocular DCNVA and DCIVA. Patient 3 showed an improvement in monocular DCIVA, while no improvement in monocular DCNVA 12 months postoperatively. Mean binocular DCNVA improved from the preoperative value of 0.41±0.10 logMAR to 0.24±0.12 logMAR after 6 months (P<0.05) and was 0.26±0.09 after 12 months (P<0.05) (Table 2, Figure 2). Mean binocular DCIVA improved from the preoperative value of 0.33±0.12 logMAR to 0.12±0.12 logMAR after 6 months (P<0.05) and 0.13±0.12 logMAR after 12 months (P<0.05) (Table 2, Figure 2).

Subjective questionnaire
Subjectively, patients reported no need for glasses for near and intermediate tasks over 6 months after surgery and were satisfied with the procedure. While at a 1-year follow-up, one patient (1/4, Patient 3) reported her dependence on glasses for near tasks such as reading books for a long time. All patients reported no changes in distance vision. Questionnaire scores ranged from three indicating extreme difficulties to zero, no difficulty. The total score was 24.5±3.1 preoperatively, 15.3±3.2 after 6 months, and 17.2±2.9 after 1 year (preoperative vs. 1 year, P<0.05).
Reading prescription
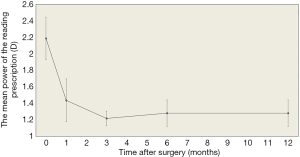
An average decrease of 0.91±0.28 D in the power of the near addition at a 40-cm reading distance was noticed and remained stable over 1 year (preoperative vs. 1 year, P<0.05) (Table 2, Figure 3).
Distance visual acuity, refraction, and stereopsis
The mean binocular UDVA was −0.10±0.08 logMAR preoperatively, −0.16±0.07 logMAR after 6 months (P>0.05), and −0.14±0.05 logMAR after 12 months (P>0.05). No statistical differences in manifest refraction were noted before and after surgery (P>0.05) (Table 2). No patients lost their CDVA postoperatively.
All subjects had a stereoacuity of 40.0 sec of arc with the Titmus test before and after surgery.
Corneal curvature, pupil size, and high order aberrations
There were no significant differences in corneal curvature and pupil sizes under photopic and mesopic lighting conditions between the preoperative and 6 months and 12 months postoperative visits (P>0.05) (Table 3). The high order aberrations, including spherical aberration, coma, and trefoil aberration, were not significantly different between preoperative and postoperative eyes (P>0.05) (Table 4).
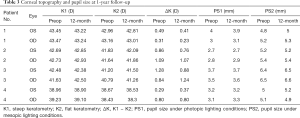
Full table
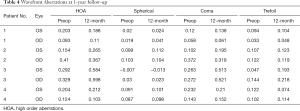
Full table
Adverse events
There was no significant change in intraocular pressure after surgery. No obvious complications except for the subconjunctival hemorrhage in one eye (Patient 1) were observed during and after surgery. Some patients experienced mild tearing, which decreased within 24 hours.
Discussion
In this prospective study, we evaluated the efficacy and safety of the LaserACE procedure for the treatment of presbyopia. After the LaserACE surgery, all patients showed an improvement in binocular near and intermediate visual acuity over the 12-month follow-up period. These patients achieved a decrease of 0.15 logMAR in DCNVA and 0.20 logMAR in DCIVA at the 12-month visit. Moreover, by measuring the reading prescription at a 40 cm reading distance, an average decrease of 0.91±0.28 D in the power of near addition was noticed. This result was comparable to those reported by Hipsley (12). In Hipsley’s study, binocular DCNVA improved from +0.21±0.17 logMAR preoperatively, to +0.11±0.12 logMAR at 24 months postoperatively. It is worth noting that DCNVA had a trend of a peak at 6 months postoperatively, and then a slight drop off between 6 and 12 months postoperatively. As for subjective satisfaction, while less than one diopter was afforded after surgery, the results of the questionnaires demonstrated that all the patients had improvement in their ability to perform near tasks without glasses. Nevertheless, one patient required glasses again 12 months postoperatively. In this regard, long-term efficacy merits observation.
The LaserACE procedure aimed to improve near and intermediate visual acuity in presbyopes by changing the rigidity of the sclera overlying the ciliary body. Ocular rigidity has been found to have an effect on age-related dysfunction of the eye (10). Hipsley proposed three critical zones of anatomical and physiological significance. After creating micropores on the sclera over these critical zones, the accommodative function can be improved. However, there remains controversy about the efficacy of scleral surgery in restoring accommodation. The most significant change contributing to presbyopia is the stiffening of the lens. Approaches targeting other accommodative structures will depend on how much restoration relies on these structures. Several studies have suggested that sclera expansion can not and does not restore accommodation (2,20-23). In comparison, Hipsley reported restoration of 1.25–1.75 D of objective accommodation after the LaserACE (11), there is little evidence so far. In a recent study, three subjects treated with LaserACE procedure up to 13 years postoperatively were studied (13). The patients displayed 0.5 D of restored accommodation and a corresponding increase in UNVA in that study, indicating a true restoration of accommodation after LaserACE. In our study, we did not analyze the objective accommodation because of the instability of the measured data. Evaluations in objective accommodation should be undertaken in future studies.
We need to consider several factors when it comes to the improvements in visual acuity and subjective accommodation after the LaserACE surgery. Firstly, the impact on accommodation is likely to be age-dependent, as dynamic methods generally attempt to make use of at least some of the still-active elements of the accommodation system. Thus, the procedure may show greater benefits for younger presbyopic patients. Secondly, those optical factors considered as pseudo-accommodation that contribute to functional near vision are important. The increase of depth of field can improve the functional near vision, including multifocality, small pupils, induced spherical aberration or astigmatism, and so on. In this study, we did not find significant changes in pupil size, spherical aberration, or corneal curvature after surgery. Therefore, these optical factors can not explain the improvement of visual function after surgery. Nonetheless, the pupil size is of value in increasing the depth of field and then improving near vision. We noticed that subject No. 3 had no improvement of DCNVA in each eye at one year follow up, whose pupil size was larger than that of the other subjects. Thirdly, improved blur interpretation, memorization, and encouragement could be partial explanations for improvements in subjective accommodation after scleral expansion surgery (21). In this concern, we eliminated the chances of memorization and controlled levels of encouragement. However, we could not completely avoid these effects.
During the 1-year visit, none of the patients showed a loss of CDVA. Changes in spherical equivalence and binocular UDVA were not statistically significant. We did not notice any complications during the one-year visit after surgery. The LaserACE can, therefore, be considered a safe procedure for the treatment of presbyopia in the emmetropic patient. Compared with other sclera approaches such as scleral implant surgery, LaserACE is less invasive and has no risk of implant erode or extrude. We also measured the wavefront aberrations before and after surgery and found no significant variations in spherical aberration and HOAs. As the cornea or the visual axis were not affected, the advantages of a scleral approach include no changes to distance vision and no glare, halo, or other optical aberrations. For this reason, it may be a safe and viable choice for emmetropic patients. Emmetropic presbyopic patients often have high expectations because they already have good distance vision and are not used to wearing glasses. Therefore, surgical outcomes both in terms of visual acuity and quality of vision are paramount in these patients.
The limitation of the study was a small sample size. As a scleral approach of presbyopic correction, the LaserACE procedure is not so widely applied and still under development. In addition, this study enrolled in emmetropic presbyopic patients. They had good distance vision and were not used to wearing glasses. These subjects often had higher expectations but less will of the surgery. Thus, we have not enrolled in a large sample of subjects. More suitable subjects will be enrolled in further studies. In statistical terms, a sample size that is too small may reduce the power of the study. Whether or not a small sample size is an important issue depends ultimately on the strength of the effect studied. Despite the small sample size, our findings provided meaningful results. Finally, the long-term stability merits further study.
Conclusions
Our findings demonstrate that the LaserACE procedure has the potential to offer a safe treatment option for presbyopia, based on the 1-year follow-up results after surgery. The mechanism and long-term effect of the procedure merit investigation. The surgical correction of presbyopia remains a significant challenge for refractive surgeons.
Acknowledgments
Funding: The study was supported in part by the National Natural Science Foundation of China (Grant No. 81770955), and Shanghai Municipal Commission of Health and Family Planning (Grant No. 20154Y0150).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2141
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2141
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2141). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Eye and ENT Hospital of Fudan University (No. ky2012-018) and informed consent was taken from all the patients after a complete description of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mantry S, Shah S. Surgical management of presbyopia. Cont Lens Anterior Eye 2004;27:171-5. [Crossref] [PubMed]
- Glasser A. Restoration of accommodation: surgical options for correction of presbyopia. Clin Exp Optom 2008;91:279-95. [Crossref] [PubMed]
- Waring GO 4th, Berry DE. Advances in the surgical correction of presbyopia. Int Ophthalmol Clin 2013;53:129-52. [Crossref] [PubMed]
- Charman WN. Developments in the correction of presbyopia II: surgical approaches. Ophthalmic Physiol Opt 2014;34:397-426. [Crossref] [PubMed]
- Gil-Cazorla R, Shah S, Naroo SA. A review of the surgical options for the correction of presbyopia. Br J Ophthalmol 2016;100:62-70. [Crossref] [PubMed]
- Greenwood M, Bafna S, Thompson V. Surgical Correction of Presbyopia: Lenticular, Corneal, and Scleral Approaches. Int Ophthalmol Clin 2016;56:149-66. [Crossref] [PubMed]
- Schachar RA. The correction of presbyopia. Int Ophthalmol Clin 2001;41:53-70. [Crossref] [PubMed]
- Croft MA, Nork TM, McDonald JP, et al. Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Invest Ophthalmol Vis Sci 2013;54:5049-58. [Crossref] [PubMed]
- Croft MA, McDonald JP, Katz A, et al. Extralenticular and lenticular aspects of accommodation and presbyopia in human versus monkey eyes. Invest Ophthalmol Vis Sci 2013;54:5035-48. [Crossref] [PubMed]
- Detorakis ET, Pallikaris IG. Ocular rigidity: biomechanical role, in vivo measurements, and clinical significance. Clin Exp Ophthalmol 2013;41:73-81. [Crossref] [PubMed]
- Hipsley A, McDonald A. Laser scleral matrix microincisions. In: Pallikaris I, Plainis S, Charman WN, editors. Presbyopia: Origins, Effects and Treatment. Slack: Thorofare, NJ, 2012. Chapter 26, p.219-25.
- Hipsley A, Ma DH, Sun CC, et al. Visual outcomes 24 months after LaserACE. Eye Vis (Lond) 2017;4:15. [Crossref] [PubMed]
- Hipsley A, Hall B, Rocha KM. Long-term visual outcomes of laser anterior ciliary excision. Am J Ophthalmol Case Rep 2018;10:38-47. [Crossref] [PubMed]
- Soloway B, Schanzlin DJ. Effect of refinements in surgical instrumentation and scleral implant device and technique on presbyopia treatment. Boston: The Annual ASCRS and ASOA Symposium and Congress, 2015.
- Lin JT, Mallo O. Treatment of presbyopia by infrared laser radial sclerectomy. J Refract Surg 2003;19:465-7. [PubMed]
- Pinelli R, Ortiz D, Simonetto A, et al. Correction of presbyopia in hyperopia with a center-distance, paracentral-near technique using the Technolas 217z platform. J Refract Surg 2008;24:494-500. [Crossref] [PubMed]
- Jung SW, Kim MJ, Park SH, et al. Multifocal corneal ablation for hyperopic presbyopes. J Refract Surg 2008;24:903-10. [Crossref] [PubMed]
- EI Danasoury AM. Gamaly TO, Hantera M. Multizone LASIK with peripheral near zone for correction of presbyopia in myopic and hyperopic eyes: 1-year results. J Refract Surg 2009;25:296-305. [Crossref] [PubMed]
- Buckhurst PJ, Wolffsohn JS, Gupta N, et al. Development of a questionnaire to assess the relative subjective benefits of presbyopia correction. J Cataract Refract Surg 2012;38:74-9. [Crossref] [PubMed]
- Qazi MA, Pepose JS, Shuster JJ. Implantation of scleral expansion band segments for the treatment of presbyopia. Am J Ophthalmol 2002;134:808-15. [Crossref] [PubMed]
- Mathews S. Scleral expansion surgery does not restore accommodation in human presbyopia. Ophthalmology 1999;106:873-7. [Crossref] [PubMed]
- Malecaze FJ, Gazagne CS, Tarroux MC, et al. Scleral expansion bands for presbyopia. Ophthalmology 2001;108:2165-71. [Crossref] [PubMed]
- Ostrin LA, Kasthurirangan S, Glasser A. Evaluation of a satisfied bilateral scleral expansion band patient. J Cataract Refract Surg 2004;30:1445-53. [Crossref] [PubMed]


