Predicting the survival rate of patients with hepatocellular carcinoma after thermal ablation by nomograms
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the fourth leading cause of cancer-related death globally (1). Thermal ablation, including radiofrequency ablation (RFA) and microwave ablation (MWA), has been widely used in clinical practice with conspicuous advantage and was suggested as the first line treatment for early HCC (2-4).
Many studies confirm that patients receiving thermal ablation have a comparable long-term outcome compared with those undergoing liver resection (5,6). However, previous studies found the recurrence rate of HCC after thermal ablation was higher than that after surgical resection (7,8). Interestingly, the fact that thermal ablation leads to a higher recurrence but a similar survival rate compared with liver resection seems to imply that the recurrence of a tumor does not affect the prognosis. However, few studies explored the connection between tumor recurrence and long-term outcomes of patients after thermal ablation.
Tumor recurrence can be classified into early and late recurrence, according to when the new lesion occurs (9,10). Usually, the occurrence of a new intrahepatic lesion within 1 or 2 years after liver resection was called early recurrence (9,11); otherwise, it was called late recurrence. Early recurrence was confirmed to be the risk factor of the long-term survival rate in patients after liver resection (11,12). Although, the risk factors of a long survival rate have been explored in other studies (13-15). Most of the earlier studies focused on pretreatment factors, including liver function, tumor number, tumor size, and AFP level (13-15). None of them mentioned the effects of early tumor recurrence on the prognosis of patients after thermal ablation. Thus, the effects of tumor recurrence on the extended survival rate in patients after thermal ablation are still unclear. In this study, the effect of early recurrence (Intrahepatic new lesion occurs within one year after ablation) on the long-term survival rate of HCC patients after thermal ablation was explored.
Nomograms have been used to estimate the individual survival rate probability. Recently, it has also been constructed to predict survival rates in patients with cancer after treatment (16,17). However, few studies used nomograms to predict prognosis for patients after thermal ablation. In this study, to unveil the connection between early recurrence and prognosis, two nomograms, taking early recurrence into account or not, were constructed to predict the long-term outcome in patients with HCC after thermal ablation. We present the following article in accordance with the TRIPOD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6116).
Methods
This retrospective study protocol was conducted in conformity with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional ethics committee of our hospital. Individual consent for this retrospective analysis was waived.
Patients with HCC undergoing percutaneous thermal ablation for radical treatment in our hospital between January 2013 and December 2018 were included in this study. The inclusion criteria were as follows: (I) patients with untreated HCC diagnosed by contrast-enhanced imaging techniques or pathology before this study, (II) patients with a single tumor with a maximum size of 5 cm or less than three tumors according to the Milan criteria, (III) patients with the liver function of Child-Pugh classification A or B, (IV) patients without portal vein tumor thrombus or extrahepatic metastasis, (V) patients with complete ablation confirmed by contrast-enhanced CT or CEMRI, (VI) patients with complete clinical data needed in this study. A total of 591 patients met the inclusion criteria and were included in this study.
The thermal ablation device, procedure, and interventional assisted techniques were described in earlier papers (4,18). All the ablative procedures were performed percutaneously by three interventional radiologists, namely XJ, JD, and YW.
Contrast-enhanced CT or MRI was performed one month after thermal ablation to confirm the complete ablation. After that, contrast-enhanced CT or MRI was performed three months after the treatment and repeated every six months. Ultrasound or contrast-enhanced ultrasound scans were performed every two to three months. Blood tests for liver function, complete blood count, and prothrombin time (PT) were routinely performed along with imaging.
Overall survival (OS) rate was calculated from the date of the treatment to the date of death or last date of follow up (survival rate or loss). The recurrence-free survival rate (RFS) rate was calculated from the date of the treatment to the date of tumor recurrence or the last date of follow-up (no finding of recurrence or loss). Tumor recurrence was defined as the occurrence of the hypervascular in the arterial phase and washed out the portal or delay phase.
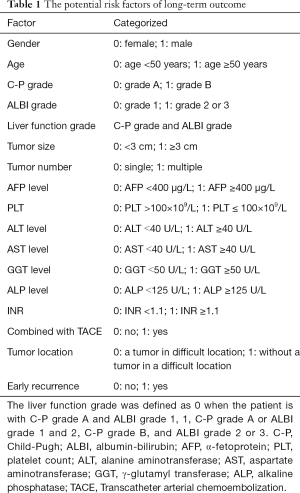
The potential risk factors, including age, gender, tumor size, tumor number, combined with Transcatheter arterial chemoembolization (TACE), tumor location, α-fetoprotein (AFP), platelet count (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), Child-Pugh (C-P) grade, albumin-bilirubin (ALBI) grade, international normalized ratio (INR) and early recurrence were collected. ALBI grade was defined as follows: [log bilirubin (µmol/L) × 0.66] + [albumin (g/L) × −0.085]; grade 1 ≤−0.260, −2.60< grade 2 <−1.39 and grade 3 ≥−1.39. The above risk factors were categorized (19,20) (Table 1).
Full table
Statistical analysis
Continuous variables are shown as mean ± standard error. Frequencies and percentages present categorical variables. Cumulative survival rate curves of OS and RFS were estimated using the Kaplan–Meier method. Cox proportional hazard models are used to identify the significant effects of risk factors for the survival rate. A P value of less than 0.05 was statistically significant. Univariate and multivariable analyses of independent prognostic factors were evaluated using the backward stepwise Cox regression model. Two sets of risk factors were shown using the Cox regression model, one excluding the early recurrence, and the other including it. All the statistical analyses were performed using the SPSS 22.0 (SPSS) software.
For two multivariate models from the Cox regression model, baseline nomogram and early recurrence-based nomograms were constructed. The performance of the model was evaluated by the concordance index (C-Index) and plotting the calibration curve. The C index was compared between the baseline and early currency-based nomograms with Z statistics. Construction of the nomogram was performed by using the Python language and the matplotlib library.
Results
Baseline characteristics
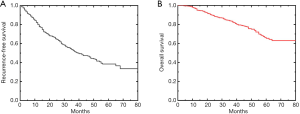
A total of 591 patients were enrolled in this study, including 407 males and 184 females, with an average age of 58.9±8.9 years. The median follow-up was 35 months (4–79 months). Before the end of the follow-up, 277 patients had tumor recurrence. The cumulative RFS rate for 1-, 2-, 3-, 4- and 5-year were 82.2%, 65%, 52.5%, 44.4% and 38.4%, respectively (Figure 1). A total of 119 patients died. The cumulative OS rate for 1-, 2-, 3-, 4- and 5-year were 96.6%, 89.2%, 83.6%, 75.5% and 65.5%, respectively (Figure 1).
Univariate and multivariate analyses
A total of 15 potential risk factors were evaluated in this study. The results of univariate analyses showed that early recurrence, tumor number, AFP level, liver function, PLT, ALP level, GGT level, AST level, and INR were the statistically significant factors. Multivariate analysis without early recurrence showed that the factors significantly affecting the OS rate were tumor number, AFP level, liver function, and GGT level. Multivariate analysis with early recurrence showed that early recurrence, tumor number, AFP level, and liver function significantly affect the OS rate (Table 2).
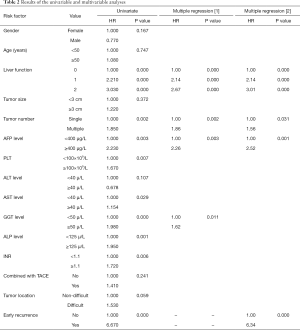
Full table
Validation and performance of Nomogram
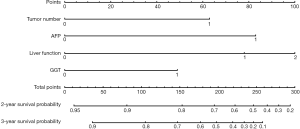
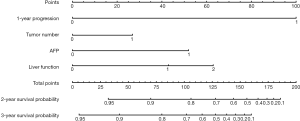
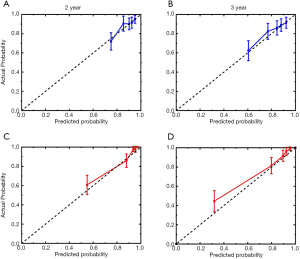
Nomogram on the tumor number, AFP level, liver function, and GGT level was called baseline nomogram (Figure 2). Another one on early recurrence, tumor number, AFP level, and liver function was called early recurrence-based nomogram (Figure 3). The C index for the model for assessment of the OS of the baseline nomogram and the early recurrence-based nomogram were 0.69 (95% CI: 0.624–0.748) and 0.81 (95% CI: 0.754–0.857), respectively, with 1,000 cycles of bootstrapping. The difference in terms of discriminating performance between the two models was significant (Z statistics =92.19, P<0.0001). Calibration curves corresponding to the 2- and 3-year survival rate matches well with the 45° line for the baseline and early recurrence-based nomogram (Figure 4).
Survival rate estimation
Using each nomogram and the survival rate estimate table, we calculated the 2- and 3-year survival rate probabilities for patients enrolled in this study. Patients were classified into different subgroups according to their different survival rate probabilities estimated by the baseline nomogram and early recurrence-based nomograms. The corresponding median survival rate of each group was calculated. All the mentioned survival rate data are shown in Tables 3 and 4. The results show with the decrease in survival rate probability, the real median survival rate reduces.
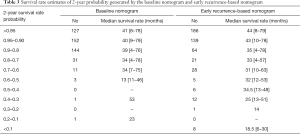
Full table
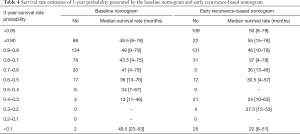
Full table
Discussion
In this study, we constructed two nomograms on prognostic models for patients with HCC undergoing thermal ablation. One of the nomograms was with the traditional risk factors of OS rate found using the Cox regression model. The other one was with the risk factors, including early recurrence. Our results showed that early recurrence has a significant effect on the survival rate of patients with HCC who were treated by thermal ablation. The nomogram taking early recurrence into account as a risk factor showed an excellent performance to estimate the survival rate probability of patients.
Survival rate and tumor recurrence are the leading indicators to assess the prognosis for patients with HCC undergoing thermal ablation (4,21,22). Both indicators gained much attention. According to the results of previous studies (5-8), patients with HCC undergoing thermal ablation have a comparable long-term survival rate but a higher tumor recurrence rate than those treated by liver resection. A confusing phenomenon that tumor recurrence does not affect the prognosis comes to us, which has not been clarified.
According to the period until recurrence, hepatic tumor recurrence can be classified into an early and late recurrence. The earlier studies (11,12) defined the early recurrence as that happening within one year after operation, and late recurrence otherwise. Other studies (9,23) defined the early recurrence as happening within two years after operation. Although the cutoff of the interval has not been well determined, the above definition seems preferable for many people. The origin of hepatic tumor recurrence is due to intrahepatic metastasis or multicentric occurrence of a new tumor in the liver remnant (10,11,23). The recurrent tumors of early recurrence may rise from intrahepatic metastasis rather than a new multicentric occurrence, which may be causally related to the neoplastic vascular infiltration, both at the macroscopic and microscopic levels. It should be noted that safe margin may also play an important role in tumor recurrence. Patients without complete ablation have a high risk of tumor recurrence and patients with sufficient ablation margin have a low risk of tumor recurrence. In the present study, only patients with complete ablation judged by contrast enhanced CECT or CEMRI were included. Moreover, the ablation margin was not calculated by fusion imaging. Thus, the risk of early and late tumor recurrence of patients with or without sufficient ablation margin was not explored in our study.
Early and late recurrences are linked to different predictive factors. The effects of early recurrence on survival rate in patients after liver resection for HCC have been reported. Poon et al. (11) reported that the three-year OS rates of patients after curative resection of HCC with and without tumor recurrence within one year were 29.7% and 48.3%, respectively. Portolani et al. (9) found the recurrence presentation modality together with the feasibility of a radical treatment are the most significant determinants for the prognosis. Shimada et al. (12) showed that the period until recurrence was a prognostic factor in patients with recurrent HCC. The early recurrence has been verified to be the risk factor of prognosis in patients after liver resection. In this study, hence, the connection of early tumor recurrence and long-term survival rate in patients with HCC after thermal ablation was explored.
Firstly, the risk factors mentioned in earlier studies were defined by the Cox proportional hazard model. We found that four factors, namely tumor number, AFP level, liver function, and GGT level, significantly affect the survival rate of patients after thermal ablation. The effect of the factors above on the survival rate has also been reported in other studies (24,25). Additionally, early recurrence, i.e., new lesions occurring within one year after thermal ablation, was enrolled as a candidate and then included in the Cox regression model. The independent risk factors of the OS have been changed to early recurrence, tumor number, AFP level, and liver function. The early recurrence instead of the GGT level becomes the statistically significant risk factor.
From the results of the Cox regression, two nomograms were constructed. The baseline nomogram used the variables readily available before treatment, namely tumor number, AFP level, liver function, and GGT level. This baseline nomogram proved a concordance index of 0.69 in the OS. The other nomogram incorporating tumor recurrence, called an early recurrence-based nomogram, has a concordance index of 0.81 in OS. The difference in terms of discriminating performance between the baseline and the early recurrence-based nomogram was significant, suggesting that the early recurrence-based nomogram predicts the probability of the survival rate of patients after thermal ablation more accurately. The survival rate after thermal ablation is usually estimated with traditional risk factors, including tumor number, tumor size, AFP level, liver function, BCLC stage, and tumor differentiation. An et al. (26) constructed a nomogram with ALBI to predict the outcome of patients after MWA with a concordance index of 0.769. A nomogram developed on tumor size, tumor number, CTP grade, platelet, and ALT to predict the local tumor progression after MWA in patients with early-stage HCC were reported to have a concordance index of 0.799 (27). However, the results of the present study show that prognosis may dynamically change on the period of tumor recurrence after thermal ablation, which is different from the previous opinions. Only using baseline risk factors to develop a prediction is insufficient.
None of the earlier studies mentioned the effects of the early recurrence on survival rate after thermal ablation. The results in the present study show that the nomogram on early recurrence significantly improves the performance of predicting the possibility of survival rate. The selected categorical variables to construct the baseline nomogram have modest hazard ratios ranging from 1.62 to 2.67. Simultaneously, early recurrence has a hazard ratio of 6.34, which is much higher than any other factor. This result shows that the early recurrence is the most powerful predictor of survival rate for patients enrolled in our study. It was not surprising that the early recurrence-based nomogram shows higher discrimination compared to the baseline one. The 2- and 3-year predicted survival rate possibilities and the corresponding median survival rate were also calculated. The results confirm that the predicted survival rate was highly consistent with the actual outcome. The survival rate possible for a particular individual can be estimated to assist physicians in making a proper therapeutic decision.
There are several limitations to our study. First, we defined the early recurrence as a recurrent tumor occurring within one year after thermal ablation. Thus, the effects of recurrent tumors occurring within two years on the survival rate of patients have not been clarified. Second, the early recurrence-based nomogram can only be used to predict the long-term outcome, and not the survival rate possibility within one year. Third, a prospective study with a large sample size is needed to verify the usefulness of this nomogram.
In conclusion, early recurrence significantly affects the long-term outcome of HCC patients undergoing thermal ablation. The nomogram with early recurrence may supply exact prognostic information for individual patients to optimize the follow-up and treatment strategy.
Acknowledgments
Funding: Tianjin Healthy Bureau supported this work (No. 2013KY04, No. 2015KY04) and Tianjin Science and Technology Commission (No. 17ZXMFSY00050, No. 17YFZCSY01070, 17ZXMFSY00170).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6116
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6116
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6116). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study protocol was conducted in conformity with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional ethics committee of our hospital. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Zhu F, Rhim H. Thermal ablation for hepatocellular carcinoma: what’s new in 2019. Chin Clin Oncol 2019;8:58. [Crossref] [PubMed]
- Bae SH, Park HC. Local modalities for inoperable hepatocellular carcinoma: radiofrequency ablation versus stereotactic body radiotherapy. Ann Transl Med 2018;6:S3. [Crossref] [PubMed]
- Ding J, Jing X, Wang Y, et al. Thermal ablation for hepatocellular carcinoma: a large-scale analysis of long-term outcome and prognostic factors. Clin Radiol 2016;71:1270-6. [Crossref] [PubMed]
- Ryu T, Takami Y, Wada Y, et al. Hepatic resection versus operative microwave ablation for single hepatocellular carcinoma ≤5 cm: A propensity score-matched analysis. Surgery 2019;166:254-62. [Crossref] [PubMed]
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;104:1775-84. [Crossref] [PubMed]
- Kim TH, Chang JM, Um SH, et al. Comparison of 2 curative treatment options for very early hepatocellular carcinoma: Efficacy, recurrence pattern, and retreatment. Medicine 2019;98:e16279. [Crossref] [PubMed]
- Liu W, Yang Z, Zou R, et al. Resection vs Ablation for Multifocal Hepatocellular Carcinomas meeting the Barcelona-Clinic Liver Cancer A Classification: A Propensity Score Matching Study. J Cancer 2019;10:2857-67. [Crossref] [PubMed]
- Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229-35. [Crossref] [PubMed]
- Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009;51:890-7. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999;229:216-22. [Crossref] [PubMed]
- Shimada M, Takenaka K, Gion T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology 1996;111:720-6. [Crossref] [PubMed]
- Yang W, Yan K, Goldberg SN, et al. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol 2016;22:2993-3005. [Crossref] [PubMed]
- Ryu T, Takami Y, Wada Y, et al. Actual 10-year survival after surgical microwave ablation for hepatocellular carcinoma: a single-center experience in Japan. Ann Surg Oncol 2019;26:4126-33. [Crossref] [PubMed]
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299-307. [Crossref] [PubMed]
- Li X, Huang H, Yu X, et al. A novel prognostic nomogram based on microvascular invasion and hematological biomarkers to predict survival outcome for hepatocellular carcinoma patients. Surg Oncol 2020;33:51-7. [Crossref] [PubMed]
- Wan G, Gao F, Chen J, et al. Nomogram prediction of individual prognosis of patients with hepatocellular carcinoma. BMC Cancer 2017;17:91. [Crossref] [PubMed]
- Ding J, Zhou Y, Wang Y, et al. Percutaneous microwave ablation of exophytic tumours in hepatocellular carcinoma patients: Safe or not? Liver Int 2017;37:1365-72. [Crossref] [PubMed]
- Hiraoka A, Michitaka K, Kumada T, et al. ALBI Score as a Novel Tool in Staging and Treatment Planning for Hepatocellular Carcinoma: Advantage of ALBI Grade for Universal Assessment of Hepatic Function. Liver Cancer 2017;6:377-9. [Crossref] [PubMed]
- Kim JH, Sinn DH, Lee JH, et al. Novel Albumin-Bilirubin Grade-Based Risk Prediction Model for Patients with Hepatocellular Carcinoma Undergoing Chemoembolization. Dig Dis Sci 2018;63:1062-71. [Crossref] [PubMed]
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900-9. [Crossref] [PubMed]
- Ma S, Ding M, Li J, et al. Ultrasound-guided percutaneous microwave ablation for hepatocellular carcinoma: clinical outcomes and prognostic factors. J Cancer Res Clin Oncol 2017;143:131-42. [Crossref] [PubMed]
- Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 2018;69:1284-93. [Crossref] [PubMed]
- Zhang L, Ge NL, Chen Y, et al. Long-term outcomes and prognostic analysis of radiofrequency ablation for small hepatocellular carcinoma: 10-year follow-up in Chinese patients. Med Oncol 2015;32:77. [Crossref] [PubMed]
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol 2007;17:684-92. [Crossref] [PubMed]
- An C, Li X, Yu X, et al. Nomogram based on albumin-bilirubin grade to predict outcome of the patients with hepatitis C virus-related hepatocellular carcinoma after microwave ablation. Cancer Biol Med 2019;16:797-810. [PubMed]
- An C, Wu S, Huang Z, et al. A novel nomogram to predict the local tumor progression after microwave ablation in patients with early-stage hepatocellular carcinoma: A tool in prediction of successful ablation. Cancer Med 2020;9:104-15. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)