
Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition)
Background
Osteoarthritis (OA) is a degenerative disease of middle-aged and elderly people caused by cartilage degeneration, fibrosis, wear and tear off, subchondral bone sclerosis, cystic degeneration, osteophyte formation at the joint margins, synovitis hyperplasia, which leads to contracture of joint capsule and ligament (1). It is characterized by articular cartilage destruction, mainly manifested as bone friction, morning stiffness, pain and joint movement disorder and so on (2). The common parts of OA involvement are the hands, knees, hips and spine, which are the main causes of pain and disability (3). OA can be divided into primary OA and secondary OA according to the etiology. At present, the cause of primary OA is not clear. Secondary OA is secondary to any joint injury or disease, such as meniscus injury, intra-articular or periarticular fracture, ligament injury, congenital deformity or dislocation, etc. The incidence of OA increased significantly with age (4), 10–17% in the population over 40 years old, 50% in the population over 60 years old, and 80% in the population over 75 years old, and the disability rate was 53% (5). The incidence of OA is higher in females than in males, and higher in rural areas than in urban areas (4). OA not only causes the decline of patients’ physical function, quality of life and social participation, but also brings huge burden to the society (6). According to statistics, by 2015, the proportion of the population over 60 years old in China was 15.5% (7). With the increase of the proportion of the elderly population, it is estimated that nearly 400 million people will suffer from OA by 2030.
In recent years, the American College of Rheumatology (ACR) (8), the European League Against Rheumatism (EULAR) (9), the American Academy of Orthopaedic Surgeons (AAOS) (10) and the international Osteoarthritis Research Society International (OARSI) (11), etc. International academic organizations have formulated or revised their own OA diagnosis and treatment guidelines. The Chinese Rheumatology Association (CRA) (5), the orthopedic Professional Committee of the Chinese Association of Integrative Medicine (12) and the joint surgery group of Chinese Orthopaedic Association (13) have also issued OA diagnosis and treatment guidelines. The above guidelines provide an important reference for the clinical diagnosis and treatment of OA in China. However, After evaluation based on the Appraisal of Guidelines for Research and Evaluation (AGREE II) (14,15) and Reporting Items for Practice Guidelines in Healthcare (RIGHT, http://www.right-statement.org) (16,17), it was found that some OA guidelines had not been registered and drafted guideline protocol, the retrieval of relevant evidence was not comprehensive, the investigation of clinical question was not conducted, the grading of evidence quality and recommendation strength was lacking, and external review and conflict of interest were not reported (Table 1). In view of this, the working group of this guideline developed the 2019 version of OA diagnosis and treatment guidelines, aims to serve as a tool for Chinese clinicians for the best decisions-making on diagnosis and treatment of OA. We present the following article in accordance with the RIGHT reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4665).
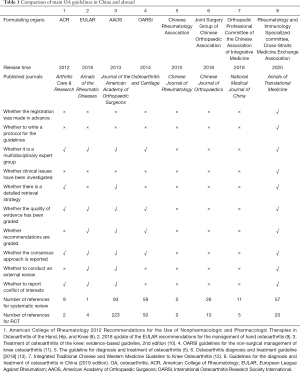
Full table
Methods
Guideline sponsors and panel members
This guideline is sponsored by the Rheumatology and Immunology Expert Committee of the Cross-Strait Medical and Health Exchange Association. The launch time was December 21, 2017, and the final date was February 23, 2019. The 6th Annual Symposium on Rheumatology and Immunology of the Cross-Strait Medical and Health Exchange Association in Beijing. This guideline has established a multidisciplinary expert group, which mainly includes experts in rheumatology, orthopedics, rehabilitation, imaging and evidence-based medicine. All panelists filled out a declaration of interest form, indicating that there are no conflicts of interest directly related to this guideline.
Guideline registration and proposal writing
This guideline has been registered on the International Practice Guidelines Registry Platform (http://www.guidelines-registry.org) (registration number IPGRP-2018CN028) (18), readers can contact the registration platform to request guideline protocol. The design and formulation of this guideline are in accordance with the “World Health Organization Handbook for Guideline Development” published in 2014 (19) and the “Basic Methods and Procedures for the Development/Revision of ‘Clinical Diagnosis and Treatment Guidelines’” (20) issued by the Chinese Medical Association in 2016. And in accordance with the AGREE II instrument and RIGHT reporting checklist.
Target users and guideline audience
This guideline is intended for rheumatologists, orthopaedic surgeons, rehabilitation physicians, clinical pharmacists, diagnostic imaging physicians, and professionals related to the diagnosis and management of OA in western medicine, integrated traditional Chinese and western medicine, and traditional Chinese medicine. The target population of the guideline is OA patients.
Selection and determination of clinical questions
The expert group used the form of questionnaire survey (21) to select clinical questions of concern to physicians. Through systematic review of published guidelines and systematic reviews in the field of OA, the working group formulated 39 clinical questions and conducted a survey of the importance of clinical questions. In the first round of investigation, 22 members of the consensus expert group were surveyed, and 66 questionnaires from the nationwide rheumatology departments were collected. After integration, a total of 28 clinical questions were collected. Based on the survey results and the discussions of the guideline working group, 16 clinical questions were finally included as questions to be addressed by this guideline.
Retrieval of evidence
The expert group deconstructed the clinical questions and outcome that were finally included according to the PICO (Population, Intervention, Comparison, and Outcome) framework, and retrieved according to the deconstructed questions: (I) MEDLINE, Cochrane Library, Epistemonikos, China Biology Medicine (CBM), Wanfang, and China National Knowledge Infrastructure (CNKI) databases, which are mainly included systematic reviews, meta-analyses, and network meta-analyses, and the search time is from inception to August 2018; (II) UpToDate, DynaMed, MEDLINE, CBM, Wanfang, and CNKI databases, mainly included original studies: randomized controlled trials (RCT), cohort studies, case-control studies, case series, epidemiological investigations, etc. The search time is from inception to November 2018; (III) National Institute for Health and Care Excellence (NICE), National Guideline Clearinghouse (NGC), official websites such as Scottish Intercollegiate Guidelines Network (SIGN), ACR, EULAR, and Asia-Pacific League of Associations for Rheumatology (APLAR), as well as MEDLINE and CNKI databases, mainly search related guidelines in the OA field; (IV) supplementary search for some other websites such as Google Scholar. Evidence was selected in the order of systematic reviews, RCTs, cohort studies, and case-control studies. This guideline finally included 58 systematic reviews and 27 RCTs.
Evaluation and classification of evidence
The expert group used the A MeaSurement Tool to Assess systematic Reviews (AMSTAR) scale (22) to conduct bias risk assessment for the included systematic reviews, meta-analyses, and network meta-analyses. Use of the Cochrane bias risk assessment tool [risk of bias (ROB), for RCTs] (23), diagnostic quality assessment tools (Quality Assessment of Diagnostic Accuracy Studies, QUADAS-2, for diagnostic accuracy tests) (24), Newcastle-Ottawa Scale (NOS, for observational studies) (25) and other methodological quality evaluation of the corresponding type of original research; the evaluation process was completed independently by two reviews. Disagreements were solved through discussion or consultaion with a third party. Use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method (26-29) to classify the body of evidence and recommendations. The certainty of the body of evidence were graded as high (Level A), moderate (Level B), low (Level C), or very low (Level D). The strength of recommendations was graded as strong (Class 1) or weak (Class 2).
Formation of recommendations
Based on the summary table of domestic and foreign evidence provided by the evidence review team, and considering the preferences and values of Chinese patients, the cost of the intervention and the balance of benefits and harms, the expert group formulated 16 recommendations. Three face-to-face consensus meetings were held in Beijing on March 9 & May 26, 2018, and Guangzhou on December 14, 2018. A total of 405 feedback comments were collected, and the expert group discussed and reviewed all recommendations and the quality of the evidence. The recommendations and draft guideline were approved by the Rheumatology and Immunology Expert Committee of the Cross-Strait Medical and Health Exchange Association.
Guideline update
This guideline plans to update the recommendations in 2022. The update method will follow the international guideline update process (30,31).
Recommendations
Clinical question: how to diagnose OA patients?
Recommendation 1: it is recommended that clinicians diagnose OA under the premise of excluding other types of joint diseases based on the main clinical manifestations such as pain in joint activity and morning stiffness (≤30 minutes) (1B)
The main clinical manifestations of OA patients include: (I) pain during joint movements, which can affect the range of joint movements in the late stages, and persistent pain and rest pain. (II) Morning stiffness: joint stiffness and tightness in the morning, can be relieved after exercise. The duration of joint stiffness in OA patients is short, generally less than 15 minutes, and a few more than 30 minutes (32). Diagnosis is generally made based on the main clinical manifestations, and imaging studies can be omitted (33). For imaging studies, ordinary X-ray examination is preferred. For further evaluation of soft tissue, it is recommended to choose ultrasound or magnetic resonance (MRI), and bone tissue examination to choose CT or MRI (33). The imaging findings of OA mainly include asymmetric joint space stenosis, subchondral bone sclerosis and/or cystic degeneration, and osteophyte formation at the joint edges. OA patients generally have no special findings in laboratory tests, and are mainly used for differential diagnosis. The OA knee, hip, hand, and shoulder classification standards released by the ACR from 1986 to 1995 combine clinical, radiological, and laboratory standards (32,34-37), and have a high sensitivity and specificity for the diagnosis of OA. The specific conditions of the patient refer to the diagnosis.
Clinical question: how to evaluate OA patients?
Recommendation 2: it is recommended that clinicians evaluate patients comprehensively based on risk factors (weight load, inflammation, metabolism, etc.), clinical manifestations and position of joint involvement (1B)
The occurrence of OA is related to many factors. Some certain occupational activities and sports may make repetitive pressure on joints and destroy articular cartilage, and then osteophyte and subchondral cyst are formed. A study published in 2017 (38) shows the risk of knee OA will be increased in people with joint weight-bearing (OR =3.29, 95% CI: 1.76, 6.15). Another study published in 2017 (39) shows the risk of hip OA in senior athletes is increased, especially in handball, football and hockey players. Analysis of 26.2 million employees in German health insurance database shows that occupations with high knee joint load is in high risk of knee OA (40). Overweight and obesity will also bring more load to the joints, especially hips and knees. The increase of joint load leads to the destruction of cartilage integrity and the reconstruction of subchondral bone, which cause in OA. A study published in 2015 (41) shows the risk of knee OA is higher in overweight people (OR =1.98, 95% CI: 1.57, 2.20) and higher in obese people (OR =2.66, 95% CI: 2.15, 3.28), and 24.6% newly diagnosed knee OA was caused by overweight or obesity. Congenital joint deformities or defects, as well as other factors resulting in bone, cartilage, ligament, meniscus and muscle damage, will lead to joint structural instability and joint biomechanical changes. Dysplasia of the hip (42), misaligned joints (43) and arched legs (44) are also risk factors for OA. Patients with knee extensor weakness (45), genu varus (46) and genu joint injury (47) also have significantly increased risks of OA. The mediators produced by joint inflammation may destroy synovium and articular cartilage which will result in OA (48). The change of metabolic environment may lead to the disorder of bone metabolism, so patients with diabetes (49), hypertension (49), gout (50), calcium pyrophosphate deposition disease (51) and hemochromatosis (52) have remarkable high risks of OA (53). Clinicians need to select the best treatment plan according to the comprehensive evaluation of these risk factors, combined with the clinical manifestations and the location of the disease.
Clinical question: what is the treatment purpose for OA patients?
Recommendation 3: the purpose of OA treatment is to relieve pain, prevent deformity, improve function and life quality (1B)
OA is a chronic degenerative disease of skeletal muscle system, which causes pain and joint dysfunction, and affects the quality of life. If it can’t be treated in time, it may cause joint deformity eventually (5). The key point of OA treatment is to relieve pain and improve joint function, so as to improve the quality of life (13,54).
Clinical question: do OA patients need to control weight?
Recommendation 4: it is recommended that OA patients should control their weight, and those who are overweight or obese should lose weight (1A)
A study published in 2016 (55) on the relationship between overweight or obesity and OA in Chinese shows the proportion of obesity in knee OA population is 2.06 times higher than that in non-knee OA population (OR =2.06, 95% CI: 1.43, 2.95), suggesting overweight or obesity is one factor influencing the development of knee OA, therefore, prevention of obesity may be a way to reduce knee OA. A study published in 2015 (56) on the relationship between body mass index and knee OA shows overweight (RR =2.45, 95% CI: 1.88, 3.20) and obesity (RR =4.55, 95% CI: 2.90, 7.13) are risk factors of knee OA, and for every 5 kg/m2 BMI increase, the risk of knee OA increases by 35%, and 24.6% newly diagnosed knee OA was caused by overweight or obesity (41). Once the patient is overweight, weight loss should be recommended. A systematic review of recommendations and guidelines for the management of OA which was published in 2014 (57) shows about half of the guidelines strongly recommend weight loss in hip or knee OA patients.
Clinical question: how do OA patients manage themselves?
Recommendation 5: it is recommended to carry out health education for OA patients, mainly to educate them about the causes, prevention, progress and treatment of the disease, reduce the burden of patients’ thoughts, and improve their self-management efficiency (1B). OA patients should reduce long-term standing, kneeling and squatting positions, ascending stairs activity, as well as bad posture, etc. (2B). It is recommended for OA patients to take reasonable joint muscle training and moderate aerobic exercise (1B). It is recommended for OA patients to choose different activities according to the location of the disease, such as grasping and holding activities of hand joints, flexion and extension activities of knee joints under the condition of non-load, and gentle activities in different directions of cervical and lumbar joints (1B)
A study published in 2014 about self-management education programs for OA (58) shows that self-management education has little or no benefit for OA patients at low to moderate evidence (MD =0.4, 95% CI: −0.39, 1.19), and compared with conventional nursing, it may reduce pain, alleviate symptoms and improve functions, but the benefit is quite rare, which is unlikely to make clinical significance. A systematic review of recommendations and guidelines for the management of OA which was published in 2014 (57) finds most guidelines have recommended self-management education and regular contact with OA patients to promote their self-care efficiency. A systematic review and meta-analysis comparing arthritis self-management education with exercise published in 2013 (59) discovers it can make better effect compare with no exercise. In order to improve the self-management efficacy of patients with knee OA, it is necessary to develop exercise intervention combined with OA self-management education plan.
The 2016 Dietary Guidelines for Chinese Residents (60) recommend at least 5 days of moderate intensity physical activity per week for more than 150 minutes in total, sticking to daily physical activities, and taking initiative 6,000 walking steps per day on average. The 2017 Ottawa guidelines for the management of knee OA (61) shows: functional aerobic exercise and intensive exercise of legs (such as cycling, hip and knee muscle strength exercises, muscle stretching and manual physical therapy, etc., twice a week, 30 minutes each time) for 4 weeks can improve patients’ physical function (62). Aerobic exercise and intensive exercise (such as fast walking, muscle stretching, 3 times a week, 1 hour each time) for 12 weeks can improve patients’ physical function (63). A 12-week bicycle exercise program (2–6 times a week, 20–60 minutes each time) can relieve joint pain, improve the physical function, and improve the quality of life (64). An 8-week Yoga Course (once a week, 60 minutes each time; plus 4 family courses every week, 30 minutes each time) (65) and an 8-week Tai Chi course (twice a week, 60 minutes each time) (66) can both improve the quality of patients’ life. A 12-week Tai Chi exercise program (once a week, 60 minutes each time) (67) and a 20-week Tai Chi exercise program (once a week, 20–40 minutes each time) (68) can both relieve pain in the knee and improve physical function of OA patients. An RCT (69) results show that Baduanjin can improve joint pain and physical function better than oral meloxicam capsule in elderly patients with knee OA. An RCT (70) results show that 12 weeks of Tai Chi, Baduanjin and bicycle training can improve the physical function of patients with knee OA better than health education, and Tai Chi and bicycle training can reduce the knee joint pain of patients. In addition, Tai Chi training can also relieve patients’ joint stiffness, improve their physical function and mental health, while Baduanjin training can improve patients’ physical function. A systemic review published in 2018 (71) shows that traditional exercise Tai Chi can relieve pain (SMD =−1.40, 95% CI: −2.28, −0.52) and improve physical function (SMD =−1.92, 95% CI: −3.16, −0.68) of knee OA patients, with few adverse reactions.
The 2017 Cochrane systematic review (72), which studies the impact of exercise on OA, shows that low-quality evidence supports that exercise can improve hand pain (SMD =−0.27, 95% CI: −0.47, −0.07), hand function (SMD =−0.28, 95% CI: −0.58, 0.02) and joint stiff (SMD =−0.36, 95% CI: −0.58, −0.15), compared to no exercise. A systematic review of the effects of aquatic exercise on muscle strength and function in OA patients in 2016 (73) showed that aquatic exercise is beneficial for improving body function, quality of life and reducing symptoms, and it is recommended that OA patients perform aquatic exercise. The results of the 2016 systematic review (74) show that high-quality evidence supports home exercise programs can reduce knee pain in patients with knee pain (SMD =0.46, 95% CI: 0.24, 0.68) and improve joint function (SMD =0.35, 95% CI: 0.15, 0.55). A 2015 Cochrane systematic review (75) of the effects of exercise on knee OA showed that land sports reduced knee pain (MD =12%, 95% CI: 10%, 15%), and improved quality of life (MD =4%, 95% CI: 2%, 5%) and improved physical function (MD =10%, 95% CI: 8%, 13%).
Clinical question: how effective and safe is topical medication for OA?
Recommendation 6: for patients with mild pain, topical application of non-steroidal anti-inflammatory drugs (NSAIDs) is recommended to reduce local pain (1B), and external application of Chinese medicine may also be considered (2B)
EULAR (76), ACR (8), OARSI (11), and NICE (77) guidelines recommend topical NSAIDs for pain relief, and topical application has a faster onset of action and a lower incidence of systemic adverse reactions, compared to oral treatment. NSAIDs for topical treatment of OA mainly include: loxoprofen, flufenamic acid, diclofenac, ketoprofen and flurbiprofen, as well as biphenylacetic acid, indomethacin, ibuprofen, nimesulide and piroxicam, etc., and they can be used externally as a solution, gel or plaster (patch) (78). A systematic review assessing the efficacy and adverse effects of topical diclofenac and ketoprofen in alleviating chronic pain in OA patients in 2016 (78) showed that diclofenac and ketoprofen can reduce pain (NNT =9.80, 95% CI: 7.10, 16.00) and (NNT =6.90, 95% CI: 5.40, 9.30) compared to placebo, and they did not increase the incidence of serious and systemic adverse reactions, but local minor adverse reactions to diclofenac increased. The RCT (79) results show that loxoprofen patch can improve the main symptoms of knee OA, and is not inferior to loxoprofen tablets.
The results of the 2012 systematic review (80) showed that the short-term efficacy and the incidence of adverse drug reactions of external treatment of knee OA with traditional Chinese medicine were similar to those of external western medicine, but significantly lower than those of oral western medicine. Multicenter RCT (81) showed that Zushi Ma plaster application can significantly reduce the pain score, improve WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) and joint function in patients with knee OA, and have many local skin adverse reactions, but the symptoms are mild and do not affect drug use. The results of RCT (82) showed that Gutong plaster combined with exercise therapy can reduce pain score and WOMAC score compared with exercise therapy alone.
Clinical question: how effective and safe is physical therapy for OA?
Recommendation 7: physical therapy such as manipulation therapy, massage, and acupuncture is recommended for OA patients to relieve pain and improve physical function (2B)
The 2016 systematic review (83) investigated the impact of manual therapy or exercise therapy on hip OA patients, and the results show that manual therapy may be beneficial for hip OA patients to reduce pain (SMD =−0.71, 95% CI: −1.08, −0.33) and physical function (SMD =−0.71, 95% CI: −1.08, −0.33). The 2013 systematic review (84) showed that manual therapy (manipulation, massage) can reduce pain and reduce disability in patients with hip OA in the short term. The results of the 2013 systematic review (85) show that manual therapy can improve joint pain and function in patients with knee OA, and has good short-term and long-term effects. The results of the 2011 systematic review (86) show that manual therapy is effective in improving short-term and long-term pain and physical function in patients with hip OA compared with exercise therapy. In summary, OA patients can use manipulation therapy, massage and other methods to relieve pain and improve physical function. The 2010 Cochrane systematic review (87) showed that acupuncture can reduce pain in patients with peripheral joint OA (SMD =−0.28, 95% CI: −0.45, −0.11) and improve patient’s physical function (SMD =−0.28, 95% CI: −0.46, −0.09). The 2016 systematic review (88) showed that acupuncture can improve short-term and long-term physical function (WMD =4.61, 95% CI: 2.24, 6.97), but pain relief is in short duration (WMD =21.24, 95% CI: 20.56, 21.92). The 2014 systematic review (89) showed that acupuncture can improve pain in patients with OA (SMD =−0.28, 95% CI: −0.45, −0.11) and physical function (SMD =−0.28, 95% CI: −0.46, −0.09). The 2013 network meta-analysis (90) showed that acupuncture can reduce pain in OA patients compared with conventional care (SMD=−0.89, 95% CI: −1.18, −0.59).
Clinical question: how effective and safe is glucosamine or chondroitin for OA?
Recommendation 8: for some patients, treatment with glucosamine or chondroitin sulfate can be selected. It should be stopped if no symptom improved after 3 to 6 months (2C)
Glucosamine and chondroitin sulfate are slow-acting OA treatment drugs, and the evidence for their treatment is still insufficient. The 2005 Cochrane systematic review (91) showed that the Rotta preparation of glucosamine can improve pain (SMD =−1.11, 95% CI: −1.66, −0.57) and functional index (SMD =−0.47, 95% CI: −0.82, −0.12). The 2007 EULAR guidelines (92) also pointed out that glucosamine and chondroitin sulfate have certain benefits in the treatment of OA, but the effect size is small, and the pathological mechanism and drug economic benefits are unclear. In 2010, a network meta-analysis (93) evaluated the efficacy of glucosamine, chondroitin sulfate, and placebo on hip or knee OA. The results showed that compared with placebo, glucosamine, chondroitin sulfate and their combination did not improve joint pain or narrowing of the joint space. A meta-analysis of the effects on hip or knee OA patients in 2000 (94) show that glucosamine and chondroitin sulfate may have a certain effect on OA, but the quality of the included studies is low, and there is a publication bias, which may exaggerate the effect. The 2005 Cochrane systematic review (91) shows that the non-Rotta preparation of glucosamine is not better than placebo and fails to improve physical function (SMD =−0.18, 95% CI: −0.31, −0.05) and relief of pain (SMD =−0.05, 95% CI: −0.15, 0.05). There is no significant difference in safety between glucosamine and placebo (RR =0.99, 95% CI: 0.91, 1.07). The 2017 Canadian Guideline (95) states that glucosamine and chondroitin supplementation can be considered if the patient has the will, and should be discontinued if there is no improvement in symptoms after 3 months of use. The 2018 network meta-analysis (96) compared long-term (≥1 year) treatment effect of 31 types of drugs, including antioxidants, bone agents, NSAIDs, intra-articular injection drugs, slow-acting drugs (glucosamine sulfate, glucosamine hydrochloride, glucosamine combined with chondroitin sulfate), and biological agents, and the results show that glucosamine sulfate (SMD =−0.29, 95% CI: −0.49, −0.09) can relieve pain for a long time, glucosamine sulfate (SMD =−0.42, 95% CI: −0.65, −0.19) and chondroitin sulfate (SMD =−0.20, 95% CI: −0.36, −0.05) can reduce joint space.
Clinical question: what is the efficacy and safety of oral NSAIDs in the treatment of OA?
Recommendation 9: for OA patients with persistent pain or moderate or severe pain, it is recommended to choose oral NSAIDs after risk assessment, and use the lowest effective dose for a short period (1–3 months) alone (1B). The combination of COX-2 inhibitor and proton pump inhibitor is recommended for patients with high risk of gastrointestinal adverse reactions (1B)
The main oral NSAIDs for the treatment of OA include: anilines: acetaminophen; xylbutans: celecoxib, etoricoxib, etc.; phenylacetic acids: diclofenac, indoleacetic acid, etc.; xicones: piroxicam, meloxicam and Lornoxicam, etc.; Propionic acids: ibuprofen, naproxen, and loxoprofen. NSAIDs such as rofecoxib, vardecoxib, and romelecoxib were delisted due to adverse events such as cardiovascular events. In terms of effectiveness, the network meta-analysis for knee and hip OA in 2017 (97) and for OA and rheumatoid arthritis in 2015 (98) evaluated the pain relief and body function improvement effects of celecoxib, naproxen, ibuprofen, diclofenac, etoricoxib and paracetamol in conventional dose. The results showed that both diclofenac 150 mg/d and etoricoxib 60 mg/d had the best pain relief effect in OA patients. In terms of the improvement of physical function, two network meta-analysis showed that (97,98) diclofenac 150 mg/d had the best effect on improving the function. Oral NSAIDs mainly have adverse reactions in gastrointestinal tract, cardiovascular system and kidney. Considering that high dose, combined use and long-term use will increase the risk of adverse reactions, one NSAIDs should be taken orally on time in a short period (1–3 months).
The incidence of gastrointestinal adverse reactions of COX-2 inhibitors is lower than that of non-selective NSAIDs. The use of proton pump inhibitors can further prevent gastrointestinal adverse reactions. The 2015 network meta-analysis (98) assessed the safety of different oral NSAIDs in the treatment of OA or rheumatoid arthritis. The incidence of major gastrointestinal adverse events relying on etoricoxib and celecoxib was lower than that of diclofenac and naproxen. In 2013, one meta-analysis (99) evaluated the gastrointestinal adverse reactions of COX-2 inhibitors with naproxen, diclofenac, ibuprofen. The results showed that COX-2 inhibitors (RR =1.81, 95% CI: 1.17, 2.81), diclofenac (RR =1.89, 95% CI: 1.16, 3.09), ibuprofen (RR =3.97, 95% CI: 2.22, 7.10), naproxen (RR =4.22, 95% CI: 2.71, 6.56) all increased gastrointestinal adverse reactions. In 2016, the network meta-analysis (100) evaluated the preventive effect of NSAIDs combined with proton pump inhibitors, histamine receptor antagonists and misoprostol on gastrointestinal adverse reactions. The results showed that the incidence of gastrointestinal adverse reactions of COX-2 inhibitors combined with proton pump inhibitors was the lowest compared with non-selective NSAIDs alone (RR =0.07, 95% CI: 0.02, 0.18), followed by COX-2 inhibitors alone (RR =0.25, 95% CI: 0.15, 0.38), Proton pump inhibitors (RR =0.28, 95% CI: 0.18, 0.41) were used in combination with non-selective NSAIDs.
NSAIDs may increase the incidence of cardiovascular adverse reactions, compared with other NSAIDs, naproxen has the lowest incidence of cardiovascular adverse reactions. The reticulated meta-analysis in 2015 (98) evaluated the incidence of major cardiovascular adverse reactions of different oral NSAIDs in the treatment of OA or rheumatoid arthritis. The results showed that the incidence of adverse reactions of naproxen in major cardiovascular events was lower than that of diclofenac, celecoxib, etoricoxib and ibuprofen, and the difference was not statistically significant. In 2013, IPD-meta analysis (99) evaluated the cardiovascular adverse reactions of COX-2 inhibitors, naproxen, diclofenac and ibuprofen. The results showed that COX-2 inhibitors increased the incidence of major vascular adverse reactions (RR =1.37, 95% CI: 1.14, 1.66), the incidence of major coronary heart disease adverse reactions (RR =1.76, 95% CI: 1.31, 2.37) and the mortality of vascular adverse reactions (RR =1.58, 95% CI: 1.00, 2.49) compared with placebo or NSAIDs. diclofenac increased the incidence of major vascular adverse reactions (RR =1.41, 95% CI: 1.12, 1.78) and adverse reactions of coronary heart disease (RR =1.70, 95% CI: 1.19, 2.41); ibuprofen increased the incidence of major adverse reactions of coronary heart disease (RR =2.22, 95% CI: 1.10, 4.48), naproxen did not increase the incidence of major vascular and adverse reactions of coronary heart disease. In 2011, the network meta-analysis (101) compared the incidence of major cardiovascular adverse reactions with naproxen, ibuprofen, diclofenac, celecoxib, etoricoxib, rofecoxib, and romelecoxib. The results showed that the incidence of myocardial infarction based on etoricoxib was the lowest, and the rate of stroke was the lowest in rofecoxib. The results showed that the incidence of myocardial infarction of etoricoxib was the lowest, the incidence of stroke of rofecoxib was the lowest, and the cardiovascular mortality, all-cause mortality and antiplatelet Trial Collaborative Group of naproxen the incidence of composite outcome was the lowest, but the difference was not statistically significant.
Clinical question: what is the efficacy and safety of traditional Chinese medicine in the treatment of OA?
Recommendation 10: for patients with OA treated with oral drugs, some oral Chinese medicine can be considered in combination (2C)
OA belongs to the category of “bone arthralgia” in traditional Chinese medicine, and the treatment of syndrome differentiation in traditional Chinese medicine has certain effect. RCTs have shown that traditional Chinese medicine can reduce pain and improve joint function in patients with knee OA. The results of multicenter RCT (102) showed that Zhuanggu joint capsule combined with celecoxib had lower WOMAC score than Zhuanggu joint capsule or celecoxib alone, and there was no significant difference in the incidence of adverse reactions. But it should be used with caution in patients with liver injury. The results of RCT (103) showed that Qufengzhitong capsule combined with Jiegu plaster could reduce the syndrome score of knee OA compared with glucosamine sulfate capsule combined with diclofenac sodium enteric coated tablets. The results of multicenter RCT (104) showed that compared with glucosamine sulfate, Gulong capsule could reduce the VAS score and WOMAC score of knee OA pain, improve the TCM syndrome score, and there was no significant difference in the incidence of adverse reactions. The results of multicenter RCT (105,106) showed that Xianlinggubao capsule could relieve pain and improve joint function compared with conventional treatment, and there was no significant difference in the incidence of adverse reactions. The results of multicenter RCT (107) showed that Wangbi tablet combined with diclofenac could relieve the symptoms and improve the joint function of knee OA.
Clinical question: what is the efficacy and safety of intra-articular injection of glucocorticoid in the treatment of OA
Recommendation 11: for patients of knee OA with persistent or moderate to severe pain, intra-articular injection of glucocorticoids is recommended for rapid relief of pain in patients with OA, the injection interval should not be shorter than 4 to 6 months (1B)
Intra-articular injection of glucocorticoid can relieve the pain in patients with OA, Especially for patients with joint cavity effusion. Solution, suspension and emulsion type can be selected for intra-articular injection. Lipid emulsion, such as Dexamethasone palmitate lipid microspheres, can effectively avoid the defects of easy precipitation of suspension and excessive absorption of solution type. Commonly used intra-articular glucocorticoids include triamcinolone, prednisolone, methylprednisolone, compound betamethasone and dexamethasone. The 2015 systematic review assessed the complementary effect and safety of intra-articular glucocorticoid in people with knee OA compared to saline, Which showed that intra-articular glucocorticoids reduce pain at 1 to 2 weeks after end of treatment (SMD =−0.48, 95% CI: −0.70, −0.27), at 4 to 6 weeks (SMD =−0.41, 95% CI: −0.61, −0.21), at 13 weeks (SMD =−0.22, 95% CI: −0.44, 0.00), and no evidence of an effect at 26 weeks (SMD −0.07, 95% CI: −0.25 to 0.11). Intra-articular corticosteroids can improve joint function at 1 to 2 weeks after end of treatment (SMD =−0.43, 95% CI: −0.72, −0.14), at 4 to 6 weeks (SMD =−0.36, 95% CI: −0.63, −0.09), and no evidence of an effect at 13 weeks or at 26 weeks. In terms of safety, there was no significant difference in the incidence of adverse effects compared with sham or no intervention. The short-term effect of intra-articular injection of glucocorticoid is better than that of hyaluronic acid (HA) (108). The 2017 systematic review compared the efficacy of intra-articular HA and intra-articular corticosteroids, which shows that pain relief in corticosteroids group decrease more than HA group up to 1 month (MD =0.67, 95% CI: 0.07, 1.27), while HA is more effective up to 6 months (MD =−0.73, 95% CI: −1.25, −0.21) (109). Repeated use of the corticosteroids can cause adverse effects, repeated injections in the same joint is not recommended, and the injection interval should not be shorter than 4 to 6 months.
Clinical question: what is the efficacy and safety of intra-articular injection of HA in the treatment of OA?
Recommendation 12: for patients of knee OA with persistent or moderate to severe pain, intra-articular injection of HA can be considered to improve the patient’s symptoms in the long term and delay the time required for joint replacement (2C)
The effect of intra-articular injection of HA on OA patients is controversial. AAOS didn’t recommend using HA for patients with symptomatic OA of the knee (110), OARSI hold that intra-articular injection of HA has a certain effect (11), and ACR keep reserved opinions (8). The 2015 network meta-analysis compared the efficacy of intra-articular HA, corticosteroids, and saline in knee OA, with oral administration of paracetamol, diclofenac, naproxen and celecoxib, IA HA performed the best analgesic effect. For function, IA HA significantly superior to IA corticosteroids and saline, For stiffness, IA HA significantly outperformed saline (111). The most commonly reported adverse effects of intra-articular injection is transient local reaction, such as joint pain and swelling, which usually subsides within a few days. However, the quality of evidence included in this systematic review is low. The 2015 AAOS systematic review only included RCTs, which showed that intra-articular injection of HA did not significantly improve pain, function and morning stiffness, and had much smaller treatment effects than trials that were not blinded. The clinical effect of intra-articular injection of HA is questionable (112). The 2015 meta-analysis evaluated the safety and efficacy of intra-articular injection of HA for knee OA. Compared to saline, intra-articular injection of hyaluronic can reduce pain at 4 to 13 weeks (SMD =0.43, 95% CI: 0.26, 0.60), and at 14 to 26 weeks (SMD =0.38, 95% CI: 0.21, 0.55), improve knee function 4 to 13 weeks (SMD =0.43, 95% CI: 0.26, 0.60), and at 14 to 26 weeks (SMD =0.38, 95% CI: 0.11, 0.45). In terms of safety, there was no significant difference in the incidence of adverse events, and no serious adverse effects were reported (113). Intra-articular injection of HA has a long duration of analgesic effect and improvement of function, with a low risk of adverse reactions, which can reduce the dosage of NSAIDs to a certain extent and prevent the long-term use of NSAIDs drugs (114). A review of systematic reviews indicates that HA is an effective intervention measure for the treatment of knee OA and will not increase the incidence of adverse reactions (115). Intra-articular injection of HA can delay the need for joint replacement and reduce medical costs.
A study of disease model for European population in 2017 indicates that intra-articular injection of HA can delay joint replacement surgery for 51–217 days and reduce medical costs by 7.5% (116). A large retrospective study of the American population in 2015 showed that patients who received one course of HA delayed total knee replacement by 0.7 years and patients who received 5 courses or more delayed total knee replacement by 3.6 years (117).
Clinical question: what is the efficacy and safety of other drugs in the treatment of OA?
Recommendation 13: for OA patients with NSAIDs contraindications or ineffective pain treatment, it is suggested to take opioids or duloxetine for analgesia (2C), or to combine diacerein, inflammatory skin extract of cowpox vaccine to inoculate of rabbits, tanezumab, technetium-99m methylene diphosphonate or bulleyaconitine A (2D)
Opioids have a certain effect on relieving the pain of OA patients. The results of the 2014 systematic review showed that opioids can improve chronic pain (SMD =−0.22, 95% CI: −0.28, −0.17)], general assessment (RD =0.13, 95% CI: 0.05, 0.21) and body function (SMD =−0.22, 95% CI: −0.28, −0.17) at 4 weeks compared to placebo, but it will also increase the withdrawal rate of adverse reactions (RD =0.17, 95% CI: 0.14, 0.21) (118). Opioids have certain addictive properties and adverse reactions. The results of the 2016 systematic review showed that there was no significant difference in pain relief between opioids and NSAIDs drugs (119). Oral opioid therapy should be carefully considered in the case of NSAIDs contraindications or treatment failure. The results of 2014 network meta-analysis showed that duloxetine performed no significant improvement in WOMAC score compared with crecoxib, naproxen, ibuprofen, etoricoxib, tramadol oxycodone, and dihydromorphone (120). The 2015 systematic review showed that duloxetine can significantly reduce pain in patients with knee OA (MD =−0.88, 95% CI: −1.11, −0.65), improve body function (MD =−4.25, 95% CI: −5.82, −2.68) and patients overall evaluation (MD =0.27, 95% CI: 0.20, 0.34), but it will increase the incidence of adverse reactions (RR =2.15, 95% CI: 1.48, 3.11) and patient dropout rates (RR =1.43, 95% CI: 1.14, 1.78) (121).
OA patients can choose to combine drugs to increase the treatment effect of OA. A network meta-analysis (122) results showed diacerein could reduce pain (UMD =−2.23, 95% CI: −2.82, −1.6) and improve physical function (UMD =−6.64, 95% CI: −10.50, −2.78) in patients with knee OA. The combined results of RCT showed that compared with celecoxib in combination with/without sodium hyaluronate the non-protein extract of inflamed rabbit skin inoculated with vaccinia virus and celecoxib in combination with/without sodium hyaluronate could increase the efficiency (123-125), improve HSS and Lysholm score (126) and reduce IL-1β, TNF-α, as well as MMP-3 levels (127,128). In the meta-analysis published in 2017 (129), Tanezumab could ameliorate knee and hip pain (MD =−0.98, 95% CI: −1.18, −0.79), improve physical function (MD =−1.10, 95% CI: −1.28, −0.92) and improve Patients’ Global Assessment score (MD =−0.27, 95% CI: −0.34, −0.20) compared with placebo in OA patients. However, adverse events, including paresthesia, arthralgia, peripheral edema, and drug discontinuation (RR =1.62, 95% CI: 1.29, 2.03) were more frequently observed in the Tanezumab group. Meanwhile, severe adverse event rates were similar between patients treated with Tanezumab and placebo. In an RCT study (130), morning stiffness duration, tender and swollen joints counts were lower among patients treated by diclofenac and (99Tc) methylene bisphosphonate injection combination compared with those treated by diclofenac monotherapy. In another RCT (131), grass carbamazepine tablets could improve pain assessment score, reduce tender and swollen joint counts and reduce WOMAC score in OA patients, which was statistically similar to diclofenac.
Clinical question: what is the efficacy and safety of intra-articular stem cell injection in OA patients?
Recommendation 14: for patients with knee OA who have poor responses with intra-articular injection of HA, stem cell injection may be considered (2D)
In recent years, stem cell injection has gradually been used for the treatment of patients with knee OA. It can be injected alone or in combination with other injection preparations. A qualitative systematic review published in 2017 (132) showed that intra-articular stem cell injection can significantly reduce pain, improve comprehensive assessment and imaging outcomes without serious adverse events. An RCT showed (133) that a single injection of stem cells could significantly improve physical function, reduce pain, and reduce the WOMAC score at 6 months. In 2019, intra-articular stem cell injection was first approved by the China Food and Drug Administration for clinical trials to treat knee OA (134).
Clinical question: what is the efficacy and safety of arthroscopic surgery in OA patients?
Recommendation 15: for knee OA patients with poor pain treatment response and mechanical symptoms, we recommend arthroscopy to reduce symptoms after assessing the risk of surgery (2C)
In 2018, the Specialized Committee of Orthopaedics and Traumatology of the Institute of Integrated Traditional Chinese and Western Medicine (12) and the Joint Surgery Group of the Chinese Orthopaedics Association (13) pointed out that arthroscopic surgery has a certain effect on knee OA with mechanical symptoms, which can clean up free body, meniscus fragments and hyperplasia. The results of a systematic review published in 2013 (135) showed that arthroscopic debridement can improve knee scores in the mid-term postoperative period (SMD =2.3, 95% CI: 1.5, 3.0). The systematic review published in 2015 evaluated the long-term effects of knee arthroscopy surgery on middle-aged and elderly patients with knee pain and degenerative knee disease (136). The results showed that no significant difference in physical function was observed between OA patients in the conservative treatment group and the arthroscopic surgery group after 2 years of following up. However, adverse events such as symptomatic deep vein thrombosis (RR =4.13, 95% CI: 1.78, 9.60), pulmonary embolism, infection and death were more frequently recorded among patients in the arthroscopic surgery group. The long-term effect of arthroscopic surgery is limited and can cause additional adverse reactions. Therefore, arthroscopic surgery should be carefully considered only in middle-aged and elderly patients with knee pain with or without signs of OA.
Clinical question: what is the efficacy and safety of arthroplasty in OA patients?
Recommendation 16: for patients with hip or knee OA who have poor response to conservative treatment and whose quality of life is significantly affected, we recommend to perform joint replacement after assessing the risk of surgery, which can relieve pain, increase the range of joint movement, and improve quality of life (1B)
The 2014 U.S. guideline (137) states that bone and joint replacement surgery has a significant impact on the quality of life of patients with hip or knee OA, such as pain, stiffness, and decreased function (based on individualized assessment of patients), and that the clinician may recommend a referral for joint replacement surgery when patients don’t respond to nonsurgical treatments. If surgery is anticipated within three months, the joint should not be injected intraarticularly in patients with hip or knee OA. The 2010 Guideline of the CRA (5) pointed out that patients with progressive OA who are over 60 years old and have poor response to medication can be replaced with joints to reduce pain and improve joint function.
A systematic review of total hip arthroplasty in 2014 (138) showed that WOMAC and hip Harris scores after hip arthroplasty were better than those before surgery, and that pain (MD =1.23, 95% CI: 0.75, 1.72), physical function (MD =1.00, 95% CI: 0.40, 1.60) and social function (MD =0.42, 95% CI: 0.04, 0.81) were improved. The results of a systematic review of hip or knee replacements in 2011 (139) showed that preoperative exercise education could improve activities after hip replacement and shorten the time to achieve standards (SMD =0.50, 95% CI: 0.10, 0.90). A systematic review of hip or knee arthroplasty published in 2015 (140) showed that early arthroplasty for patients with hip or knee OA can shorten the duration of hospital stay, improve range of motion, muscle strength and quality of life. A systematic review of the outcomes of joint replacement surgery published in 2016 (141) showed that in patients undergoing joint replacement surgery, surgery could improve early postoperative pain and WOMAC function scores.
Limitations
The guideline should be considered following limitations: first, the evidence retrieval was completed in December 2018 and the guideline may not have incorporated some latest evidence. Second, the working group did not conduct systematic review and some of the systematic reviews and RCTs included were of low quality.
Research gaps
Based on the recommendations and evidence of this guideline, we have identified the following research gaps to guide future research:
❖ How to evaluate and classify OA patients by high applicability tools for targeted therapy?
❖ What is the effectiveness and safety of stem cell injection for the treatment of patients with knee OA?
❖ How does long-term effect of the perform joint replacement for OA patients?
Acknowledgments
We thank the members of evidence review team for involving the work of evidence review (Nan Yang, Jingyi Zhang, Yanfang Ma, Jianjian Wang, Xiao Liu, Qi Zhou, Xufei Luo).
Funding: This guideline was supported by: (I) China National Centre for Biotechnology Development, Ministry of Science and Technology of China (No. 2019YFC1709805); (II) National Key R&D Program of China (No. 2018YFC1705503).
Footnote
Reporting Checklist: The authors have completed the RIGHT reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4665
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4665). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965-73. [Crossref] [PubMed]
- Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet 2015;386:376-87. [PubMed]
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323-30. [Crossref] [PubMed]
- Tang X, Wang S, Zhan S, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol 2016;68:648-53. [Crossref] [PubMed]
- Chinese Rheumatology Association. The guideline for diagnosis and treatment of osteoarthritis. Chin J Rheumatol 2010;14:416-9.
- Liu Y, Wang Y. The economics of osteoarthritis. Chin J Osteoporos 2011;17:181-4.
- National Bureau of Statistics of People's Republic of China. China Statistical Yearbook. Beijing: China Statistics Press, 2015.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken) 2012;64:465-74. [Crossref] [PubMed]
- Kloppenburg M, Kroon FP, Blanco FJ, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis 2019;78:16-24. [Crossref] [PubMed]
- Jevsevar DS. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline, 2nd Edition. J Am Acad Orthop Surg 2013;21:571-6.
- McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363-88. [Crossref] [PubMed]
- Joint Surgery Group, Chinese Orthopaedics Association. Integrated Traditional Chinese and Western Medicine Guideline to Knee Osteoarthritis. Natl Med J China 2018;98:3653-8.
- Joint Surgery Group, Chinese Orthopaedics Association. Osteoarthritis diagnosis and treatment guideline (2018). Chin J Orthop 2018;38:705.
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42. [Crossref] [PubMed]
- Wei D, Wang CY, Xiao XJ, et al. Interpretation of Guideline Research and Evaluation Tools (AGREEII). Chin J Evid Based Pediatr 2013;8:316-9.
- Chen Y. Study on Reporting Norms of Health Care Practice Guidelines. Lanzhou University, 2015.
- Chen Y, Yang KH, Marušić A, et al. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med 2017;166:128-32. [Crossref] [PubMed]
- Evidence-based Medicine Center of Lanzhou University/ Chinese GRADE Center. International Practice Guideline Registry Platform. Available online: http://www.guidelines-registry.cn/index.php?m=content&c=index&a=page_project&guestid=196
- World Health Organization. WHO Handbook for Guideline Development. Second edition. 2014. Available online: https://apps.who.int/medicinedocs/en/m/abstract/Js22083en/
- Jiang Z, Zhan S, Jia X, et al. Basic methods and procedures for formulating / revising the Clinical Diagnosis and Treatment Guidelines. Natl Med J China 2016;96:250.
- Xing D, Wang Q, Yang Z, et al. Evidence‐based guidelines for intra‐articular injection in knee osteoarthritis: Formulating and evaluating research questions. Int J Rheum Dis 2018;21:1533-42. [Crossref] [PubMed]
- Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Wells GA, Shea BJ, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute 2013:1-4.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. [Crossref] [PubMed]
- Chen YL, Yang KH, Yao L, et al. Advances in GRADE system methodology. Chin J Evid Based Pediatr 2013;8:64-5.
- Chen YL, Yao L, Norris S, et al. Application of GRADE in Systematic Reviews: Necessity, Frequently-Asked Questions and Concerns. Chin J Evid-Based Med 2013;13:1401-4.
- Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744. [Crossref] [PubMed]
- Becker M, Neugebauer EA, Eikermann M. Partial updating of clinical practice guidelines often makes more sense than full updating: a systematic review on methods and the development of an updating procedure. J Clin Epidemiol 2014;67:33-45. [Crossref] [PubMed]
- Chen Y, Wang X, Wu Q, et al. Survey on Update Condition of Clinical Practice Guidelines in China. Chin J Evid-Based Med 2014;(2):178-83.
- Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039-49. [Crossref] [PubMed]
- Sakellariou G, Conaghan PG, Zhang W, et al. EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann Rheum Dis 2017;76:1484-94. [Crossref] [PubMed]
- Altman RD. The classification of osteoarthritis. J Rheumatol Suppl 1995;43:42-3. [PubMed]
- Altman RD. Criteria for the classification of osteoarthritis of the knee and hip. Scand J Rheumatol Suppl 1987;65:31-9. [Crossref] [PubMed]
- Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990;33:1601-10. [Crossref] [PubMed]
- Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 1991;34:505-14. [Crossref] [PubMed]
- Ren Y, Shi YY, Tan B, et al. Meta-analysis of the risk factors for knee osteoarthritis among the Chinese population. Modern Prevent Med 2015;42:2282-4.
- Vigdorchik JM, Nepple JJ, Eftekhary N, et al. What is the association of elite sporting activities with the development of hip osteoarthritis? Am J Sports Med 2017;45:961-4. [Crossref] [PubMed]
- Liebers F, Brendler C, Latza U. Age- and occupation-related differences in sick leave due to frequent musculoskeletal disorders. Low back pain and knee osteoarthritis. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:367-80. [Crossref] [PubMed]
- Silverwood V, Blagojevic-Bucknall M, Jinks C, et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:507-15. [Crossref] [PubMed]
- Terjesen T. Residual hip dysplasia as a risk factor for osteoarthritis in 45 years follow-up of late-detected hip dislocation. J Child Orthop 2011;5:425-31. [Crossref] [PubMed]
- Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum 2009;61:459-67. [Crossref] [PubMed]
- Chapple CM, Nicholson H, Baxter GD, et al. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken) 2011;63:1115-25. [Crossref] [PubMed]
- Øiestad BE, Juhl CB, Eitzen I, et al. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:171-7. [Crossref] [PubMed]
- Sharma L, Chang AH, Jackson RD, et al. Varus thrust and incident and progressive knee osteoarthritis. Arthritis Rheumatol 2017;69:2136-43. [Crossref] [PubMed]
- Muthuri SG, McWilliams DF, Doherty M, et al. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage 2011;19:1286-93. [Crossref] [PubMed]
- Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006;20:3-25. [Crossref] [PubMed]
- Zhang YM, Wang J, Liu XG. Association between hypertension and risk of knee osteoarthritis: A meta-analysis of observational studies. Medicine 2017;96:e7584. [Crossref] [PubMed]
- Bevis M, Marshall M, Rathod T, et al. The association between gout and radiographic hand, knee and foot osteoarthritis: a cross-sectional study. BMC Musculoskelet Disord 2016;17:169. [Crossref] [PubMed]
- Stucki G, Hardegger D, Böhni U, et al. Degeneration of the Scaphoid-Trapezium Joint: A Useful Finding to Differentiate Calcium Pyrophosphate Deposition Disease from Osteoarthritis. Clin Rheumatol 1999;18:232-37. [Crossref] [PubMed]
- Pierce TP, Issa K, Ramirez A, et al. Ochronosis as etiology of requiring total knee arthroplasty-a case series. Surg Technol Int 2016;29:261-64. [PubMed]
- Wassenaar MJE, Biermasz NR, Duinen NV, et al. High prevalence of arthropathy, according to the definitions of radiological and clinical osteoarthritis, in patients with long-term cure of acromegaly: A case-control study. Eur J Endocrinol 2009;160:357-65. [Crossref] [PubMed]
- Chinese Orthopaedics Association. Osteoarthritis diagnosis and treatment guideline (2007). Chin J Orthop 2007;27:28-30.
- Gao X, Huang S, Zhang Y, et al. A systematic review of the correlation between overweight/obesity and osteoarthritis in Chinese population. Chin J Obes Metab Dis 2016;2:164-9. (Electronic Edition).
- Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open 2015;5:e007568. [Crossref] [PubMed]
- Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum 2014;43:701-12. [Crossref] [PubMed]
- Kroon FPB, van der Burg LRA, Buchbinder R, et al. Self‐management education programmes for osteoarthritis. Cochrane Database Syst Rev 2014.CD008963. [PubMed]
- Brand E, Nyland J, Henzman C, et al. Arthritis self-efficacy scale scores in knee osteoarthritis: a systematic review and meta-analysis comparing arthritis self-management education with or without exercise. J Orthop Sports Phys Ther 2013;43:895. [Crossref] [PubMed]
- Chinese Nutrition Society. Chinese residents diet guideline. Available online: http://dg.cnsoc.org/.2018-12-03
- Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs. Clin Rehabil 2017;31:612-24. [Crossref] [PubMed]
- Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med 2000;132:173-81. [Crossref] [PubMed]
- Péloquin L, Bravo G, Gauthier P, et al. Effects of a cross-training exercise program in persons with osteoarthritis of the knee a randomized controlled trial. J Clin Rheumatol 1999;5:126-36. [Crossref] [PubMed]
- Salacinski AJ, Krohn K, Lewis SF, et al. The effects of group cycling on gait and pain-related disability in individuals with mild-to-moderate knee osteoarthritis: a randomized controlled trial. J Orthop Sports Phys Ther 2012;42:985-95. [Crossref] [PubMed]
- Cheung C, Wyman JF, Resnick B, et al. Yoga for managing knee osteoarthritis in older women: a pilot randomized controlled trial. BMC Complement Altern Med 2014;14:160. [Crossref] [PubMed]
- Lee HJ, Park HJ, Chae Y, et al. Tai Chi Qigong for the quality of life of patients with knee osteoarthritis: a pilot, randomized, waiting list controlled trial. Clin Rehabil 2009;23:504-11. [Crossref] [PubMed]
- Fransen M, Nairn L, Winstanley J, et al. Physical activity for osteoarthritis management: a randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Rheum 2007;57:407-14. [Crossref] [PubMed]
- Tsai PF, Chang JY, Beck C, et al. A pilot cluster-randomized trial of a 20-week Tai Chi program in elders with cognitive impairment and osteoarthritic knee: effects on pain and other health outcomes. J Pain Symptom Manage 2013;45:660-9. [Crossref] [PubMed]
- Wang C, Meng L. Clinical Study on Baduanjin Intervention for Elderly Knee Osteoarthritis. Cardiovascular Disease Electronic Journal of Integrated Traditional Chinese and Western Medicine 2016;4:158.
- Hu K. A Randomized Controlled Study on the Intervention Effect of Different Exercise Methods on Middle-aged and Aged Knee Osteoarthritis Patients in the Community. Fujian University of Traditional Chinese Medicine, 2017.
- Zeng L, Yang W, Guo D, et al. System evaluation of traditional exercise therapy intervention on pain and joint function improvement in patients with knee osteoarthritis. China J Tradit Chin Med Pharm 2018.2132-9.
- Østerås N, Kjeken I, Smedslund G, et al. Exercise for Hand Osteoarthritis: A Cochrane Systematic Review. J Rheumatol 2017;44:1850-8. [Crossref] [PubMed]
- Mattos F, Leite N, Pitta A, et al. Effects of aquatic exercise on muscle strength and functional performance of individuals with osteoarthritis: a systematic review. Rev Bras Reumatol Engl Ed 2016;56:530-42. [Crossref] [PubMed]
- Anwer S, Alghadir A, Brismée JM. Effect of home exercise program in patients with knee osteoarthritis: a systematic review and meta-analysis. J Geriatr Phys Ther 2016;39:38-48. [Crossref] [PubMed]
- Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med 2015;49:1554-7. [Crossref] [PubMed]
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145. [Crossref] [PubMed]
- National Clinical Guideline Centre (UK). Osteoarthritis: Care and Management in Adults. London: National Institute for Health and Care Excellence (UK); 2014.
- Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2016;4:CD007400. [Crossref] [PubMed]
- Mu R, Bao CD, Chen ZW, et al. Efficacy and safety of loxoprofen hydrogel patch versus loxoprofen tablet in patients with knee osteoarthritis: a randomized controlled non-inferiority trial. Clin Rheumatol 2016;35:165-73. [Crossref] [PubMed]
- Xu YP, Xie LM, Wang WY. Meta-analysis on efficacy and safety of external use of tradition Chinese medicines and western medicines in treating knee osteoarthritis. Zhongguo Zhong Yao Za Zhi 2012;37:2977. [PubMed]
- Jiao J, Huang CB, Wang HD, et al. Effect of zushima plaster on knee osteoarthritis: multicenter randomized-controlled trial. Chin J Allerg Clin Immunol 2018;12:283-8.
- Xu J, Wang G, Xue R, et al. A Randomized Control Clinical Study on Gutong Plaster Combined with Exercise Therapy in Treatment for Knee Osteoarthritis. Chinese Journal of Medicinal Guide 2015;(12):1265-9.
- Sampath KK, Mani R, Miyamori T, et al. The effects of manual therapy or exercise therapy or both in people with hip osteoarthritis: A systematic review and meta-analysis. Clin Rehabil 2016;30:1141-55. [Crossref] [PubMed]
- Romeo A, Parazza S, Boschi M, et al. Manual therapy and therapeutic exercise in the treatment of osteoarthritis of the hip: a systematic review. Reumatismo 2013;65:63. [Crossref] [PubMed]
- Wang Q, Zhu G. Therapeutic effect of manipulation therapy on knee osteoarthritis: a systematic review. Shanghai J Tradit Chin Med 2013;(11):11-5.
- French HP, Brennan A, White B, et al. Manual therapy for osteoarthritis of the hip or knee – A systematic review. Man Ther 2011;16:109-17. [Crossref] [PubMed]
- Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev 2010.CD001977. [PubMed]
- Lin X, Huang K, Zhu G, et al. The Effects of Acupuncture on Chronic Knee Pain Due to Osteoarthritis: A Meta-Analysis. J Bone Joint Surg Am 2016;98:1578-85. [Crossref] [PubMed]
- Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med 2014;14:312. [Crossref] [PubMed]
- Corbett M S, Rice S J, Madurasinghe V, et al. Acupuncture and other physical treatments for the relief of pain due to osteoarthritis of the knee: network meta-analysis. Osteoarthritis Cartilage 2014;22:712-3. [Crossref] [PubMed]
- Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2005.CD002946. [PubMed]
- Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:377-88. [Crossref] [PubMed]
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010;341:c4675. [Crossref] [PubMed]
- McAlindon TE, Lavalley MP, Gulin JP, et al. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA 2000;283:1469-75. [Crossref] [PubMed]
- Kielly J, Davis EM, Marra C. Practice guidelines for pharmacists: The management of osteoarthritis. Can Pharm J (Ott) 2017;150:156-68. [Crossref] [PubMed]
- Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: A systematic review and meta-analysis. JAMA 2018;320:2564-79. [Crossref] [PubMed]
- da Costa BR, Reichenbach S, Keller N, et al. RETRACTED: Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2016;387:2093-105. [Crossref] [PubMed]
- van Walsem A, Pandhi S, Nixon RM, et al. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res Ther 2015;17:66. [Crossref] [PubMed]
- Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769-79. [Crossref] [PubMed]
- Yuan JQ, Tsoi KK, Yang M, et al. Systematic review with network meta-analysis: comparative effectiveness and safety of strategies for preventing NSAID-associated gastrointestinal toxicity. Aliment Pharmacol Ther 2016;43:1262-75. [Crossref] [PubMed]
- Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. [Crossref] [PubMed]
- Zhang XL, Yang J, Yang L, et al. Efficacy and Safety of Zhuanggu Joint Capsules in Combination with Celecoxib in Knee Osteoarthritis: A Multi-center, Randomized, Double-blind, Double-dummy, and Parallel Controlled Trial. Chin Med J 2016;129:891-7. [Crossref] [PubMed]
- Xu JH, Xu B, Deng YQ. Efficacy of the self-made external bone-knitting ointment combined with the dispelling wind to relieve pain capsule on the treatment of knee osteoarthritis. Chin J Osteoporos 2014;(10):1202-6.
- Tang X, Jiang Q, Liu P. A Multicenter Randomized Controlled Clinical Study of Gulong Capsule for Knee Osteoarthritis. Chin J New Drug 2018;21:1865-71.
- Wang F, Shi L, Zhang Y, et al. A Traditional Herbal Formula Xianlinggubao for Pain Control and Function Improvement in Patients with Knee and Hand Osteoarthritis: A Multicenter, Randomized, Open-Label, Controlled Trial. Evid Based Complement Alternat Med 2018;2018:1827528. [Crossref] [PubMed]
- Zhu HM, Qin L, Garnero P, et al. The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis. Osteoporos Int 2012;23:1317-27. [Crossref] [PubMed]
- Kang XZ, Wu QF, Jie HY. Clinical Study on the Treatment of Knee Osteoarthritis by Wangbi Tablet. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011;31:1205-8. [PubMed]
- Jüni P, Hari R, Rutjes AWS, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015;10:CD005328. [PubMed]
- He WW, Kuang MJ, Zhao J, et al. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: A meta-analysis. Int J Surg 2017;39:95. [Crossref] [PubMed]
- Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013;21:577-9.
- Bannuru RR, Schmid CH, Kent DM, et al. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis: A Systematic Review and Network Meta-analysis. Ann Intern Med 2015;162:46-54. [Crossref] [PubMed]
- Jevsevar D, Donnelly P, Brown GA, et al. Viscosupplementation for Osteoarthritis of the Knee: A Systematic Review of the Evidence. J Bone Joint Surg Am 2015;97:2047-60. [Crossref] [PubMed]
- Strand V, Mcintyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res 2015;8:217-28. [PubMed]
- Altman RD, Devji T, Bhandari M, et al. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: A systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum 2016;46:151-9. [Crossref] [PubMed]
- Xing D, Wang B, Liu Q, et al. Intra-articular Hyaluronic Acid in Treating Knee Osteoarthritis: a PRISMA-Compliant Systematic Review of Overlapping Meta-analysis. Sci Rep 2016;6:32790. [Crossref] [PubMed]
- Delbarre A, Amor B, Bardoulat I, et al. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis - A Cox model analysis. PLoS One 2017;12:e0187227. [Crossref] [PubMed]
- Altman R, Lim S, Steen RG, et al. Hyaluronic Acid Injections Are Associated with Delay of Total Knee Replacement Surgery in Patients with Knee Osteoarthritis: Evidence from a Large U.S. Health Claims Database. PLoS One 2015;10:e0145776. [Crossref] [PubMed]
- da Costa BR, Nüesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2014.CD003115. [Crossref] [PubMed]
- Smith SR, Deshpande BR, Collins JE, et al. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: Systematic analytic review. Osteoarthritis Cartilage 2016;24:962-72. [Crossref] [PubMed]
- Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord 2014;15:76. [Crossref] [PubMed]
- Wang ZY, Shi SY, Li SJ, et al. Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain:A Meta-analysis of Randomized Controlled Trials. Pain Med 2015;16:1373-85. [Crossref] [PubMed]
- Kongtharvonskul J, Anothaisintawee T, Mcevoy M, et al. Efficacy and safety of glucosamine, diacerein, and NSAIDs in osteoarthritis knee: a systematic review and network meta-analysis. Eur J Med Res 2015;20:24. [Crossref] [PubMed]
- Cao DG, Feng SQ, Zhou XH, et al. Observation of the therapeutic effect of neurotropin combined with celecoxib on knee osteoarthritis. Proceedings of the 6th Chinese Journal of Orthopaedics Forum. General Hospital of Tianjin Medical University 2013:185-9.
- Cao D, Feng S, Zhou X, et al. Clinical observation of neurotropin combined with celecoxib in the treatment of knee osteoarthritis. Chinese Journal of Bone and Joint 2014;(4):292-6.
- Gong JH, Wang HJ, Song DM, et al. Observation of the efficacy of sodium hyaluronate combined with celecoxib and neurotropin in the treatment of knee osteoarthritis. Chinese Journal of Clinical Rational Drug Use 2015;(28):87-8.
- Gong JH, Wang HJ, Song DM, et al. Clinical analysis of 60 cases of knee osteoarthritis. Chinese Journal of Clinical Rational Drug Use 2015;(31):87-8.
- Gong JH, Wang HJ, Song DM, et al. Effects of combined drug therapy on knee osteoarthritis on IL-1β, TNF-α and MMP-3 levels of patients' knee joint fluid. Chinese Journal of Clinical Rational Drug Use 2015;(34):72-3.
- Shi ZM. Therapeutic effect of drug combination on the levels of IL-1β, TNF-α and MMP-3 in knee joint fluid of patients with knee osteoarthritis. Chinese Journal of Health Care Nutrition 2017;(17):367-8.
- Chen J, Li J, Li R, et al. Efficacy and Safety of Tanezumab on Osteoarthritis Knee and Hip Pains: A Meta-Analysis of Randomized Controlled Trials. Pain Medicine 2017;18:374-85. [PubMed]
- Hu B, Meng Z, He B, et al. Clinical research about 99Tc-MDP treatment osteoarthritis. Gansu Medical Journal 2015;(1):31-3.
- Xu N, Liu R, Zang JJ, et al. Efficacy and safety of bulleyaconitine A on patients with osteoarthritis. China Medicine 2014;9:1170-3.
- Pas HI, Winters M, Haisma HJ, et al. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med 2017;51:1125. [Crossref] [PubMed]
- Lee WS, Kim HJ, Kim KI, et al. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med 2019;8:504-11. [Crossref] [PubMed]
- Center for drug evaluation, NMPA. Drug clinical trial approval (ID: CXSL1800109). Available online: =changePage&pageName=servicehttp://www.cde.org.cn/news.do?method
- Spahn G, Hofmann GO, Klinger HM. The effects of arthroscopic joint debridement in the knee osteoarthritis: results of a meta-analysis. Knee Surg Sports Traumatol Arthrosc 2013;21:1553. [Crossref] [PubMed]
- Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ 2015;350:h2747. [Crossref] [PubMed]
- Non-Surgical Management of Hip and Knee Osteoarthritis Working Group. VA/DoD clinical practice guideline for the non-surgical management of hip and knee osteoarthritis. Washington (DC): Department of Veterans Affairs, Department of Defense; 2014:126.
- Shan L, Shan B, Graham D, et al. Total hip replacement: a systematic review and meta-analysis on mid-term quality of life. Osteoarthritis Cartilage 2014;22:389-406. [Crossref] [PubMed]
- Wallis JA, Taylor NF. Pre-operative interventions (non-surgical and non-pharmacological) for patients with hip or knee osteoarthritis awaiting joint replacement surgery--a systematic review and meta-analysis. Osteoarthritis Cartilage 2011;19:1381-95. [Crossref] [PubMed]
- Guerra ML, Singh PJ, Taylor NF. Early mobilization of patients who have had a hip or knee joint replacement reduces length of stay in hospital: a systematic review. Clin Rehabil 2015;29:844-54. [Crossref] [PubMed]
- Wang L, Lee M, Zhang Z, et al. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2016;6:e009857. [Crossref] [PubMed]


