Evaluation of methods for H. pylori detection in PPI consumption using culture, rapid urease test and smear examination
Introduction
H. pylori has been regarded as an important risk factor for development of chronic gastritis, peptic ulcers, gastric cancer and mucosa-associated lymphoid tissue lymphomas (1). H. pylori infection has been reported as 80% in developing and 20-50% in developed countries (2). High prevalence of H. pylori in some regions of the world is associated with high incidence of severe gastric diseases such as peptic ulcers in China (17.2%) (3) or gastric cancer in developed (61.4%) and developing (64.4%) countries (4). According to previous studies in Iran, the prevalence of H. pylori ranges from 69% in the general population (5) to 89.2% in high-incidence areas of gastric cancer, Ardabil and Meshkinshahr in North-West of Iran (6,7). The high rate of mortality from gastric cancer, 18 per 100,000 (8) and considerable number of referrals to endoscopy units due to complaint of dyspepsia indicate the need for appropriate antimicrobial therapies for cure of patients suffering from peptic ulcers (7) or those who are first-degree relatives of gastric cancer patients (9). Furthermore, considerable resistance rates of H. pylori to currently-used antibiotics (metronidazole: 55.6%, clarithromycin: 7.3%, amoxicillin: 7.3%, tetracycline 38.1% and furazolidone: 4.5%) (10) indicate the need for performance of antimicrobial susceptibility test. In this regard, endoscopy provides gastric biopsy samples for H. pylori diagnosis and antibiogram, while evaluating the degree of gastric lesions.
Diagnostic tests for H. pylori infection have been classified into two groups; those that are performed before antimicrobial therapy and those performed after, for confirmation of eradication .The former includes culture, histology, rapid urease test (RUT), histopathology, PCR and serology and the latter; urea breath test (UBT) and stool antigen test (SAT) (11). Culture is the most specific method for detection of H. pylori infection (12). Growth of H. pylori from biopsy cultures was the turning point in etiology and treatment of gastric diseases (13). Culturing success depends on the quality of biopsy specimen, the gastric site of biopsy, appropriate transport of the biopsy to microbiology lab and it’s processing in a short time (14,15). The sensitivity and specificity of culture for diagnosis of H. pylori in experienced laboratories has been reported as 50-92% (16-21) and 100% (16,17), respectively. Culture of H .pylori from the gastric mucosa by string test has been reported, although with lower sensitivity (22). Positive results of H. pylori culture from feces have also been demonstrated (23).
Smear examination or imprint cytology has been used as a simple, rapid and inexpensive method to diagnose H. pylori infection and decide for antimicrobial therapy in endoscopy room (19,24). The typical spiral H. pylori cells could be observed in stained smears of gastric biopsies when examined by light microscopy (25,26). Imprint cytology by Diff-Quick staining method has high sensitivity (82%) and specificity (100%) comparable with histopathologic methods (27). RUT is a highly specific, rapid and simple method with low cost. The time needed for obtaining the results ranges from few minutes to 24 hr, depending on the bacterial density in the biopsy and their urease activity (11). It has been proposed that for positive RUT result, more than 10,000 bacterial cells are required. False-negative results of RUT could occur due to decrease or interruption of urease activity after consumption of proton pump inhibitors (PPIs), antibiotics or bismuth salts (28). Commercial RUT kits have the sensitivity of 85-90% and specificity >95-100% (29).
Histopathology is an accurate method for H. pylori diagnosis (30). Microscopic examination of fixed and stained biopsy specimens provides critical information about the severity of inflammation and atrophic changes in gastric epithelium (11,19). However, good quality biopsy and highly experienced pathologist are needed for obtaining accurate results (31). Low bacterial number (32), bacterial absence (33) and uneven distribution of bacterial cells could lead to false-negative results (18). Accordingly, the sensitivity and specificity of this method vary between 50-100%, depending on the quality of biopsy specimen and bacterial density (34). UBT is the measurement of exhaled CO2 released due to H. pylori urease activity in the gastric mucosa, with the sensitivity and specificity of >90%. It is easy to perform without the need for endoscopy (35). UBT has been regarded as an excellent method for detection of the moderate bacterial density with patchy distribution, which might happen after antimicrobial therapy. Post-treatment UBT can be performed 4-6 weeks after termination of antimicrobial therapy (36). UBT can also be used for H. pylori diagnosis in adults and children, using non-radioactive C13 (37). However, false-negative results could occur due to consumption of drugs such as PPI that affect H. pylori growth or urease activity (38). SAT is an enzyme-linked immunoassay method that detects H. pylori antigens in stool samples with the sensitivity and specificity of >90% (39,40). Diagnosis accuracy of SAT for active H. pylori infection in adults was similar to UBT, however, it showed lower detection rate when used for post-treatment diagnosis (41). SAT has been regarded as an accurate and non-invasive test for diagnosis of H. pylori infection in children before antimicrobial therapy (37) and for confirmation of eradication after therapy (40).
Some authors believe that none of the diagnostic tests for H. pylori is the gold standard and results of more than one test could be acceptable for confirming bacterial infection (19). However, others propose that culture is the gold standard for diagnosis of H. pylori infection and is the only method that makes isolation of the bacterium and studying it in detail, possible (15). Culture, compared with other methods that are practically simpler, faster and easier to perform, needs experienced personnel and is time-consuming (42). However, it is clear that culture of gastric biopsies yields a great amount of H. pylori for further studies such as isolation and identification of the bacterium, performance of antimicrobial susceptibility test, establishment of a biobank for research and discovery of other possible gastric microorganisms. Several reports have indicated that although culture is an accurate method, false-negative results could occur due to consumption of PPIs which have inhibitory effect on H. pylori growth (43) and urease activity (44). In this study the prevalence of H. pylori infection was determined in 530 dyspeptic patients; using gastric biopsies for culture, RUT and smear examination. The efficacy of culture for diagnosis of H. pylori infection was compared with RUT and smear examination. The effect of PPI consumption on the results of three tests was also evaluated.
Methods
Patients
The study was performed on 530 unselected dyspeptic patients (male: 243, 45.5% and female: 291, 54.5%), aged 14-92 years, who were referred to the endoscopy room of Digestive Disease Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Informed consent obtained from each patient and the study was approved by the Research Ethics Committee of Tehran University of Medical Sciences. Patients were divided into two groups according to PPI consumption; patients with PPI consumption until 1-2 days before endoscopy and those consuming no PPI for at least 4 weeks before endoscopy. Two antral biopsy specimens were obtained from each patient. One for RUT and the second for culture and smear examination which was placed in sterile semi-solid transport medium (NaCl 0.9%, bacteriological agar 0.16%) and transferred to microbiology laboratory before 3 hr.
Rapid urease test (RUT)
RUT was performed in endoscopy room by placing one antral biopsy into 0.5 mL urea solution in an Eppendorf tube, containing urea (2%) phosphate buffer (0.25 M) and phenol red (12 mg/L) as pH indicator (pH ≤6.8) (Bahar afshan Co., Iran). Positive result was recorded as change in color from yellow to pink and negative as no color change. The time for color change ranged from few minutes to 24 hr. Accordingly, RUT results were interpreted as those which become positive within 1 hr and those up to 24 hr (45).
Culture and smear examination
Antral biopsies were surface inoculated on the selective brucella blood agar containing 7% defibrinated sheep blood, vancomycin (5 mg/L), trimethoprim (5 mg/L), polymyxin B (50 µg/L), and amphotericin B (4 mg/L). Cultured plates were incubated at 37 °C under microaerobic conditions with high humidity. Cultures were examined after 3-5 days of incubation for observing pinpoint glistening colonies. Negative cultures were further incubated and observed up to 2 weeks. Bacterial strains were identified as H. pylori on the basis of Gram stain and spiral microscopic appearance as well as positive activities of urease, oxidase, and catalase (10). After culturing, the remaining biopsy was evenly smeared on the glass slide. Biopsy smears were Gram-stained and visualized by light microscopy for observing Gram-negative spiral bacterial cells (19). Negative results were those in which H. pylori cells were not observed up to 60 min of microscopic searching.
Statistical analyses
Statistical analysis was performed using the Pearson’ chi-square test and the Fisher’s exact probability test (IBM SPSS version.20) to determine the association between PPI consumption and results of culture, RUT or smear examination. A statistically significant level was defined as P≤0.05. Considering positive culture as gold standard, sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV) and likelihood ratio for RUT was calculated. Due to considerable number of unexamined smears, sensitivity and specificity of culture were calculated only on the basis of positive result of RUT.
Results
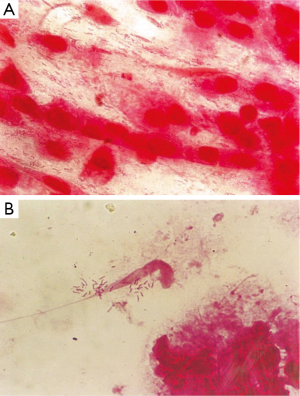
From 530 patients studied; 242 (45.6%) were male and 288 (54.4%) female, 14-92 (mean age: 46±16.4) yr. Of total number of patients, 473 (89.3%) were diagnosed having gastritis and 57 (10.7%) peptic ulcer. PPI was consumed by 326 (61.5%) and not by 204 (39.5%). Biopsy culture of 214 (40.4%) patients was positive and RUT of 256 (48.3%) turned positive within few minutes up to 24 hr. Out of 234 smears examined, H. pylori was observed in 112 (47.9%), mostly as clades of tens of spiral bacteria (Table 1, Figure 1). The overall H. pylori infection rate was estimated 54% when at least one positive test was taken as an indicator of H. pylori occurrence.
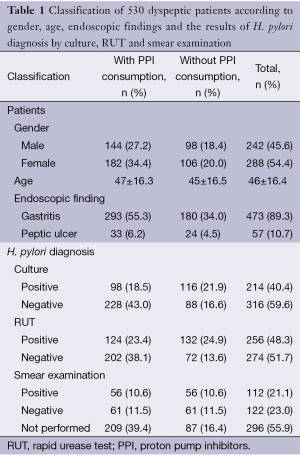
Full table
Out of 530 patients studied, results of H. pylori culture were consistent with those of RUT in 438 (82.6%). Among 189 (35.7%) patients with positive results of culture and RUT, 80 (15.1%) consumed PPI and 109 (20.6%) did not. Among 249 (47%) patients with negative results of culture and RUT, 184 (34.7%) consumed PPI and 65 (12.3%) did not. A strong correlation was found between PPI consumption and negative results of both culture and RUT (P value =0). Among 189 patients with positive results of culture and RUT, smear of 171 (32.3%) patients were observed and 97 (18.3%) indicated the presence of H. pylori cells. Among these patients, 46 (8.7%) consumed PPI and 51 (9.6%) did not. Among 249 patients with negative results of culture and RUT, 5 (0.9%) had positive smear, 3 (0.6%) consumed PPI and 2 (0.4%) did not. Results of H. pylori culture did not agree with those of RUT in 92 (17.4%) patients. Biopsy samples of 25 (4.7%) patients were culture positive but RUT negative while 67 (12.6%) samples were culture negative but RUT positive. Among these patients 62 (11.7%) consumed PPI and 30 (5.6%) did not. In patients with disagreement of culture and RUT, 10 (1.9%) patients had positive smear, 28 (5.2%) had negative and 54 (10.2%) were not examined (Table 2). Statistical analysis was performed for those patients with one positive results of at least one test. The number of patients with PPI consumption and negative results of culture or RUT was considerably high, showing significant correlation between negative results of culture (47, 16.4%) or RUT (21, 7.3%) and PPI consumption (P<0.05). This correlation was not observed in the analysis of smear results showing that PPI consumption could have a negative impact on the results of H. pylori culture and RUT but not on smear (Table 3).
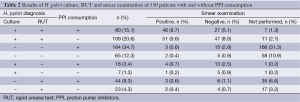
Full table
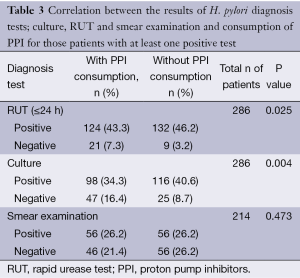
Full table
On the basis of RUT results, sensitivity and specificity of culture in patients who consumed PPI were 64.5% and 91%, respectively, compare with patients who consumed no PPI (82.5% and 90.2%). Sensitivity and specificity of smear examination in patients who consumed PPI were 55.9% and 77.7%, respectively, compared with patients who consumed no PPI (50% and 69.2%) but the results were not reliable due to considerable number of unexamined smears. On the basis of culture results, sensitivity, specificity, PPV, NPV and likelihood ratio of 24-hr and 1-hr RUT were calculated. In patients who consumed PPI, sensitivity, specificity, PPV, NPV, positive likelihood ratio and negative likelihood ratio of 1-hr were 74.4%, 93.8%, 83.9%, 89.5%, 5.2 and 0.1 and those of 24-hr RUT were 81.6%, 80.7%, 64.5%, 91%, 1.8 and 0.1. In patients without PPI consumption, sensitivity, specificity, PPV, NPV, positive likelihood ratio and negative likelihood ratio of 1-hr RUT were 92.2%, 85.2%, 89.1%, 89.2%, 8.2 and 0.1 and those of 24-hr were 93.9%, 73.8%, 82.5%, 90.2%, 4.7 and 0.1 (Table 4).
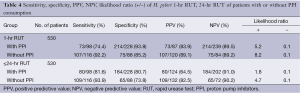
Full table
Discussion
The prevalence of H. pylori among 530 patients was estimated as 40.4% by culture (sensitivity: 64.5-82.5%), 48.3% by RUT (sensitivity: 74.4-93.9%) and 21.1% by smear examination (sensitivity: 50-77.7%) and the overall H. pylori infection rate was 54%. Out of 234 Gram-stained smears, spiral bacterial cells were visualized in 112 (47.9%) samples; 97 culture and RUT (+), 5 culture (−) and RUT (+), 5 culture (+) and RUT (−) and 5 culture and RUT (−). Detection rate of culture was lower than those reported by others (50-70%) (20,21). Our results of Gram-stained smear examination were consistent with those from Kuwait in which 184/252 (67.4%) smears examined by imprint cytology method were positive for the presence of H. pylori cells. Furthermore, from 270 smears stained by Gram’s Method, 52.2% showed H. pylori cells. In the same study H. pylori culture was positive in 43.7% of 261 cultured biopsies and RUT turned positive in 56.4% of 264 biopsy samples (19). In Taiwan, H. pylori was detected in 30.4% of patients by culture, 28.9% by RUT and 36.2% by histopathology. The sensitivity of culture was 91.46%, histopathology 95.12% and RUT 64.63% (antrum) and 69.51% (corpus) (18). In a study from Bangladesh, H. pylori was detected in 49.5% of patients by smear examination (sensitivity; 86.2%), 45.6% by haematoxylin & eosin staining (sensitivity; 77.6%), 51.5% by modified Giemsa staining (sensitivity; 86.4%) and 56.4% by RUT (sensitivity; 96.6%) (26).
Out of 530 gastric biopsies studied, 80 biopsies were positive for culture and RUT despite PPI consumption, indicating that culture and RUT might not be affected by PPI consumption in some patients, although the number of patients with negative results of culture and RUT who consumed PPI (184, 34.7%) was 2.8 times more than those who did not (65, 12.3%) (P value =0). Statistical analyses on patients with at least one positive test showed a significant correlation between negative results of culture or RUT and PPI consumption (P value <0.05). This correlation was not observed in the analysis of smear examination results, showing that PPI consumption could have a negative impact on the results of H. pylori culture and RUT but not on smear examination.
The sensitivity and specificity of RUTs have been reported as a range of 88-93% and 99-100%, respectively. It has been indicated that at 1hr, the sensitivity of CLO test reduced to 66-71%. However, reading the results longer than 1 hr increased the accuracy of the test, proposing that increasing the incubation time may improve the sensitivity of RUT but decreases its specificity (45,46). Good performance of urease test has been demonstrated with the sensitivity of 89.6%, specificity 100%, PPV 100% and NPV 84.1% (18). In this study, on the basis of culture results, the 24-hr RUT had higher sensitivity than 1-hr RUT in patients who consumed PPI. There was no significant difference between the sensitivity of 24-hr and 1-hr RUT in patients without PPI consumption. The sensitivity of 24-hr RUT was reduced from 93.9% to 81.6% as the result of PPI consumption. This change in sensitivity was more profound in 1-hr RUT, which showed a reduction from 92.2% to 74.4% due to PPI consumption. Our results showed that in patients who consumed no PPI, 1-hr RUT has the best performance with the sensitivity of 92.2%, specificity 85.2%, PPV 89.1%, NPV 89.2%, positive likelihood ratio 8.2 and negative likelihood ratio 0.1. These results are consistent with those of other study which showed the sensitivity of 1-hr RUT as 39% in patients with PPI consumption and 78% in those without. Moreover, the 24-hr RUT was found as 62% in patients with PPI consumption and 89% in those without (47).
It has been demonstrated that PPIs and bismuth exhibit bactericidal activity against H. pylori (43). PPIs cause rise in the stomach pH, leading to accumulation of ammonia produced by H. pylori urease and suppression of bacterial viability (48). PPIs and antibiotics cause spiral forms of H. pylori to turn into coccoid with lower urease activity (49). Chen et al. found no difference in diagnosis accuracy of H. pylori infection by multiplex-PCR in patients with (74%) or without (75%) PPI consumption and indicated that in patients with PPI consumption, PCR had higher detection rate (74%) than RUT (18%) and histology (50%) (50). In vitro studies showed the concentration-dependent inhibitory effect of PPI on H. pylori growth (43) and its virulence properties (51). Lansoprazole exhibited bactericidal activity at ≤0.625 µg/mL which was 4-fold and 16-fold higher than omeprazole and pantoprazole, respectively. The inhibitory effect of lansoprazole on RUT was twice more than omeprazole and 6 times than pantoprazole (52). These results indicate that the effect of PPI on H. pylori culture and RUT could depend on the kind and dosage of PPI consumed by patients.
Result of this study showed an overall detection rate of 54% by culture, RUT and smear, which was considerably lower compared with results of previous studies from Iran, obtained by serology, 69-89% (5,6) or histopathology and RUT, 89.2% (7). Several reports indicate decrease in the prevalence of H. pylori in some parts of the world. It has been proposed that this decline could be the result of public awareness, improvement of personal hygiene, living conditions and diagnostic methods as well as effective eradication of H. pylori (53). However, others suggest that one important reason for reported decrement of H. pylori prevalence could be false negative results of H. pylori diagnostic tests due to failure in bacterial growth and urease activity after PPI consumption (43,51).
Decline in the positive results of culture, RUT or smear examination could be due to several other factors such as: wrong site of biopsy sampling, small size of the biopsy, destruction of biopsy during endoscopy, low bacterial density in biopsy, consumption of alcohol or histamine H2-receptor blockers (54) or antibacterial compounds released from neutrophils during tissue processing (55). Several recommendations for improving the results of culture, RUT and smear examination have been proposed. Using multiple biopsies (56), mincing and homogenizing biopsies before culturing (57), longer incubation time (58), maintaining high level of humidity during incubation (59), wiping the anaerobic jars with 70% alcohol (59) or using copper sulfate solution in water tray of incubators to postpone the fungal growth.
Although culture is a tedious and time-consuming method for recovery of H. pylori from gastric epithelium, it provides plenty of bacterial cells for studying their phenotypic and genotypic characteristics while allowing evaluation of antimicrobial resistance. Furthermore, culture provides the opportunity for discovery of new microorganisms that inhabit the stomach. We have isolated two unique mucoid H. pylori stains with high resistance to antibiotics that could withstand exposure to O2 for a long time, compared with the control H. pylori strain (60). We also isolated Candida yeasts from gastric biopsies and studied the intracellular occurrence of H. pylori inside the vacuole of yeast (61,62). Several reports indicate isolation of different microorganisms from human stomach, including Streptococci (63), Lactobacilli (64) and yeast (65).
Culture, RUT and smear examination are the most accurate methods used for H. pylori diagnosis when good quality biopsies with moderate to high bacterial density are recruited. However, among the diagnostic tests for H. pylori infection, culture and RUT are mostly affected by PPI consumption, yielding false negative results. PPI prescription has been reported considerably high in some countries (66), even for mild dyspepsia (67). PPIs are also highly prescribed in Iran, mainly for subclinical to mild gastritis, leading to false negative results of H. pylori diagnostic tests, including RUT in endoscopy room. Accordingly, consumption of PPIs should be stopped at least 2-4 weeks before performing the endoscopy (68).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Versalovic J. Helicobacter pylori. Pathology and diagnostic strategies. Am J Clin Pathol 2003;119:403-12. [PubMed]
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175-86. [PubMed]
- Li Z, Zou D, Ma X, et al. Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in China. Am J Gastroenterol 2010;105:2570-7. [PubMed]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030-44. [PubMed]
- Nouraie M, Latifi-Navid S, Rezvan H, et al. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter 2009;14:40-6. [PubMed]
- Sadjadi A, Malekzadeh R, Derakhshan MH, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer 2003;107:113-8. [PubMed]
- Barazandeh F, Yazdanbod A, Pourfarzi F, et al. Epidemiology of peptic ulcer disease: endoscopic results of a systematic investigation in iran. Middle East J Dig Dis 2012;4:90-6. [PubMed]
- Babaei M, Pourfarzi F, Yazdanbod A, et al. Gastric cancer in Ardabil, Iran--a review and update on cancer registry data. Asian Pac J Cancer Prev 2010;11:595-9. [PubMed]
- Siavoshi F, Asgharzadeh A, Ghadiri H, et al. Helicobacter pylori genotypes and types of gastritis in first-degree relatives of gastric cancer patients. Int J Med Microbiol 2011;301:506-12. [PubMed]
- Siavoshi F, Saniee P, Latifi-Navid S, et al. Increase in resistance rates of H. pylori isolates to metronidazole and tetracycline--comparison of three 3-year studies. Arch Iran Med 2010;13:177-87. [PubMed]
- Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, et al. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol 2014;20:1438-49. [PubMed]
- van Zwet AA, Thijs JC, Kooistra-Smid AM, et al. Sensitivity of culture compared with that of polymerase chain reaction for detection of Helicobacter pylori from antral biopsy samples. J Clin Microbiol 1993;31:1918-20. [PubMed]
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311-5. [PubMed]
- Ndip RN, MacKay WG, Farthing MJ, et al. Culturing Helicobacter pylori from clinical specimens: review of microbiologic methods. J Pediatr Gastroenterol Nutr 36:616-22. [PubMed]
- Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006;19:449-90. [PubMed]
- Ramis IB, de Moraes EP, Fernandes MS, et al. Evaluation of diagnostic methods for the detection of Helicobacter pylori in gastric biopsy specimens of dyspeptic patients. Braz J Microbiol 2012;43:903-8. [PubMed]
- Hirschl AM, Makristathis A. Methods to detect Helicobacter pylori: from culture to molecular biology. Helicobacter 2007;12:6-11. [PubMed]
- Lee HC, Huang TC, Lin CL, et al. Performance of Routine Helicobacter pylori Invasive Tests in Patients with Dyspepsia. Gastroenterol Res Pract 2013;2013:184806.
- Al-Ali J, Al-Asfar F, Dhar R, et al. Diagnostic performance of gastric imprint smear for determination of Helicobacter pylori infection. Can J Gastroenterol 2010;24:603-6. [PubMed]
- Grove DI, Koutsouridis G, Cummins AG. Comparison of culture, histopathology and urease testing for the diagnosis of Helicobacter pylori gastritis and susceptibility to amoxycillin, clarithromycin, metronidazole and tetracycline. Pathology 1998;30:183-7. [PubMed]
- Loffeld RJ, Stobberingh E, Flendrig JA, et al. Helicobacter pylori in gastric biopsy specimens. Comparison of culture, modified giemsa stain, and immunohistochemistry. A retrospective study. J Pathol 1991;165:69-73. [PubMed]
- Perez-Trallero E, Montes M, Alcorta M, et al. Non-endoscopic method to obtain Helicobacter pylori for culture. Lancet 1995;345:622-3. [PubMed]
- Thomas JE, Gibson GR, Darboe MK, et al. Isolation of Helicobacter pylori from human faeces. Lancet 1992;340:1194-5. [PubMed]
- Misra SP, Dwivedi M, Misra V, et al. Imprint cytology--a cheap, rapid and effective method for diagnosing Helicobacter pylori. Postgrad Med J 1993;69:291-5. [PubMed]
- Pinto MM, Meriano FV, Afridi S, et al. Cytodiagnosis of Campylobacter pylori in Papanicolaou-stained imprints of gastric biopsy specimens. Acta Cytol 1991;35:204-6. [PubMed]
- Akanda MR, Rahman AN. Comparative Study of Different Methods for Detection of Helicobacter Pylori in Gastric Biopsies. Dinajpur Med Col J 2011;4:1-6.
- Kaur G, Madhavan M, Basri AH, et al. Rapid diagnosis of Helicobacter pylori infection in gastric imprint smear. Med J Malaysia 2002;57:67. [PubMed]
- Lewis JD, Kroser J, Bevan J, et al. Urease-based tests for Helicobacter pylori gastritis. Accurate for diagnosis but poor correlation with disease severity. J Clin Gastroenterol 1997;25:415-20. [PubMed]
- Tseng CA, Wang WM, Wu DC. Comparison of the clinical feasibility of three rapid urease tests in the diagnosis of Helicobacter pylori infection. Dig Dis Sci 2005;50:449-52. [PubMed]
- Glupczynski Y. The diagnosis of Helicobacter pylori infection: a microbiologist’s perspective. Reviews in Medical Microbiology 1994;5:199-208.
- Mégraud F. Advantages and disadvantages of current diagnostic tests for the detection of Helicobacter pylori. Scand J Gastroenterol 1996;31:57-62. [PubMed]
- Korstanje A, van Eeden S, Offerhaus GJ, et al. The 13carbon urea breath test for the diagnosis of Helicobacter pylori infection in subjects with atrophic gastritis: evaluation in a primary care setting. Aliment Pharmacol Ther 2006;24:643-50. [PubMed]
- Satoh K, Kimura K, Taniguchi Y, et al. Biopsy sites suitable for the diagnosis of Helicobacter pylori infection and the assessment of the extent of atrophic gastritis. Am J Gastroenterol 1998;93:569-73. [PubMed]
- El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney System. Hum Pathol 1999;30:72-7. [PubMed]
- Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther 2004;20:1001-17. [PubMed]
- Shirin H, Levine A, Shevah O, et al. Eradication of Helicobacter pylori can be accurately confirmed 14 days after termination of triple therapy using a high-dose citric acid-based 13C urea breath test. Digestion 2005;71:208-12. [PubMed]
- Leal YA, Cedillo-Rivera R, Simón JA, et al. Utility of stool sample-based tests for the diagnosis of Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr 2011;52:718-28. [PubMed]
- Goddard AF, Logan RP. Review article: urea breath tests for detecting Helicobacter pylori. Aliment Pharmacol Ther 1997;11:641-9. [PubMed]
- Korkmaz H, Kesli R, Karabagli P, et al. Comparison of the diagnostic accuracy of five different stool antigen tests for the diagnosis of Helicobacter pylori infection. Helicobacter 2013;18:384-91. [PubMed]
- Guo YY, Zhang ST, Peng XX, et al. A systematic review of diagnosis of Helicobacter pylori infection by Helicobacter pylori stool antigen test. Zhonghua Yi Xue Za Zhi 2005;85:1564-7. [PubMed]
- Veijola L, Oksanen A, Löfgren T, et al. Comparison of three stool antigen tests in confirming Helicobacter pylori eradication in adults. Scand J Gastroenterol 2005;40:395-401. [PubMed]
- Mégraud F. How should Helicobacter pylori infection be diagnosed? Gastroenterology 1997;113:S93-8. [PubMed]
- Iwahi T, Satoh H, Nakao M, et al. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother 1991;35:490-6. [PubMed]
- Yakoob J, Jafri W, Abbas Z, et al. The diagnostic yield of various tests for Helicobacter pylori infection in patients on acid-reducing drugs. Dig Dis Sci 2008;53:95-100. [PubMed]
- Laine L, Lewin D, Naritoku W, et al. Prospective comparison of commercially available rapid urease tests for the diagnosis of Helicobacter pylori. Gastrointest Endosc 1996;44:523-6. [PubMed]
- Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev 1997;10:720-41. [PubMed]
- Dickey W, Kenny BD, McConnell JB. Effect of proton pump inhibitors on the detection of Helicobacter pylori in gastric biopsies. Aliment Pharmacol Ther 1996;10:289-93. [PubMed]
- Weil J, Bell GD, Powell K, et al. Omeprazole and Helicobacter pylori: temporary suppression rather than true eradication. Aliment Pharmacol Ther 1991;5:309-13. [PubMed]
- Andersen LP, Dorland A, Karacan H, et al. Possible clinical importance of the transformation of Helicobacter pylori into coccoid forms. Scand J Gastroenterol 2000;35:897-903. [PubMed]
- Chen T, Meng X, Zhang H, et al. Comparing Multiplex PCR and Rapid Urease Test in the Detection of H. pylori in Patients on Proton Pump Inhibitors. Gastroenterol Res Pract 2012;2012:898276.
- Nakao M, Tada M, Tsuchimori K, et al. Antibacterial properties of lansoprazole alone and in combination with antimicrobial agents against Helicobacter pylori. Eur J Clin Microbiol Infect Dis 1995;14:391-9. [PubMed]
- Nakao M, Malfertheiner P. Growth inhibitory and bactericidal activities of lansoprazole compared with those of omeprazole and pantoprazole against Helicobacter pylori. Helicobacter 1998;3:21-7. [PubMed]
- Tan HJ, Goh KL. Changing epidemiology of Helicobacter pylori in Asia. J Dig Dis 2008;9:186-9. [PubMed]
- Leszczyńska K, Namiot A, Namiot Z, et al. Patient factors affecting culture of Helicobacter pylori isolated from gastric mucosal specimens. Adv Med Sci 2010;55:161-6. [PubMed]
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532-5. [PubMed]
- Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc 1994;40:342-5. [PubMed]
- Cellini L, Allocati N, Campli ED, et al. Helicobacter pylori isolated from stomach corpus and antrum: comparison of DNA patterns. J Infect 1996;32:219-21. [PubMed]
- Glupczynski Y. Microbiological and serological diagnostic tests for Helicobacter pylori: an overview. Br Med Bull 1998;54:175-86. [PubMed]
- Owen RJ. Bacteriology of Helicobacter pylori. Baillieres Clin Gastroenterol 1995;9:415-46. [PubMed]
- Siavoshi F, Saniee P, Atabakhsh M, et al. Mucoid Helicobacter pylori isolates with fast growth under microaerobic and aerobic conditions. Helicobacter 2012;17:62-7. [PubMed]
- Siavoshi F, Saniee P. Vacuoles of Candida yeast as a specialized niche for Helicobacter pylori. World J Gastroenterol 2014;20:5263-73. [PubMed]
- Siavoshi F, Nourali-Ahari F, Zeinali S, et al. Yeasts protects Helicobacter pylori against the environmental stress. Arch Iran Med 1998;1:2-8.
- Zilberstein B, Quintanilha AG, Santos MA, et al. Digestive tract microbiota in healthy volunteers. Clinics (Sao Paulo) 2007;62:47-54. [PubMed]
- García A, Sáez K, Delgado C, et al. Low co-existence rates of Lactobacillus spp. and Helicobacter pylori detected in gastric biopsies from patients with gastrointestinal symptoms. Rev Esp Enferm Dig 2012;104:473-8. [PubMed]
- Zwolinska-Wcisło M, Budak A, Bogdał J, et al. Fungal colonization of gastric mucosa and its clinical relevance. Med Sci Monit 2001;7:982-8. [PubMed]
- IMS-Health. 2009 US sales and prescription information. 2010. Available online: http://imshealth.com
- Roberts SJ, Bateman DN. Prescribing of antacids and ulcer-healing drugs in primary care in the north of England. Aliment Pharmacol Ther 1995;9:137-43. [PubMed]
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007;56:772-81. [PubMed]


