Mannose-binding lectin activation is associated with the progression of diabetic nephropathy in type 2 diabetes mellitus patients
Introduction
Diabetes accounts for 30–50% of chronic kidney disease (CKD) and the proportion is increasing rapidly (1). Renal inflammation is involved in the pathogenesis and progression of diabetic nephropathy (DN) (2-5). Mannose-binding lectin (MBL) is a natural immune soluble medium that was shown to be involved in the development of DN (6,7). Zheng et al. reported that MBL might act in the activation of the complement mediated renal interstitial injury (8). Li et al. found that MBL and factor Bb levels in serum and urine were correlated with the severity of DN (9). Also, MBL deposit was found in renal pathology in patients with type 2 diabetes mellitus (DM) in different populations (10).
In this study, we aimed to determine whether MBL contributes to the progression of DN and to identify its correlation with the endpoint of end-stage renal disease (ESRD) in DN patients; single nucleotide polymorphisms (SNPs) of MBL2 gene were also studied. We present the paper in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1073).
Methods
Patients
We enrolled 77 type 2 DM patients who were admitted into our center from August 2013 to September 2016. All of them received a renal biopsy, and serum and urine sample acquisition at the time of renal biopsy. This retrospective cohort study has adhered to the STROBE guidelines for cohort studies. The diagnosis of type 2 DM or DN was according to the criteria proposed by the American Diabetes Association (ADA) in 2017 (11) or the criteria by the Renal Pathology Society (RPS) in 2010 (12), respectively. Patients complicated with other kidney diseases, active infections, severe liver dysfunction, under immunosuppressive therapy, kidney allograft recipients, reaching ESRD before renal biopsy, with incomplete baseline data, or lost in follow-up were excluded. The flow chart is shown in Figure 1. The number of cases during the study period determined the sample size. The research protocols were conformed to the provisions of the Declaration of Helsinki (as revised in 2013) and were approved by the Ethics Committee of the First Affiliated Hospital of Medical School of Zhejiang University (No. 2017243). All patients were informed consent.
The baseline characteristics of the patients including the demographic data, physical examination, the comprehensive chemical panel on the day of the renal biopsy were collected from the hospital information system. We kept following up each patient until a confirmed ESRD or the last follow-up time of August 31, 2018. The data on outcomes was obtained from outpatient medical records, the website of Zhejiang Medical Quality Control and Evaluation for Dialysis, and telephone follow-ups. ESRD was defined as an estimated glomerular filtration rate (eGFR, calculated by CKD-EPI formula) lower than 15 mL/min/1.73m2 or requirement of renal replacement therapy.
Blood and urine samples processing
Blood and urine samples were collected on the same day of renal biopsy. Serum and blood mononuclear cells were separated and stored in aliquots at −80 °C until usage. Serum samples for complement measurement were thawed at room temperature for 1 hour, and then centrifuged at 3,500 rpm for 5 min at 4 °C in a Thermo ScientificTM HeraeusTM MultifugeTM X1 Centrifuge (ThermoFisher Scientific, Rockford, IL, USA).
Quantification of MBL levels
Serum or urine MBL levels were measured by enzyme linked immunosorbent assay (ELISA). Commercial ELISA kits (R&D Systems, USA) were used according to the manufacturer’s instructions. Samples were thawed rapidly at 37 °C and then kept on ice before measuring to ensure no additional complement activation. Repeated freeze-thaw cycles were avoided.
Renal histology
Each biopsy was routinely processed for light microscopy, immunofluorescence, and electron microscopy. The pathological classification and scores were evaluated based upon the 2010 RPS criteria. Renal pathological findings were categorized by glomerular lesions (I, IIa, IIb, III, IV), interstitial fibrosis and tubular atrophy (IFTA) [0, 1, 2, 3], interstitial inflammation [0, 1, 2] and arteriosclerosis [0, 1, 2].
Cell sample processing
Cell samples were thawed, transferred to ethylenediaminetetraacetic acid (EDTA) tubes, remained at room temperature for 30 min, and then centrifuged at 3,500 rpm for 5 min at 4 °C in a Thermo ScientificTM HeraeusTM MultifugeTM X1 Centrifuge (Thermo-Fisher Scientific, Rockford, IL, USA). The supernatant was disposed with erythrocyte lysis and then centrifuged again at 3,500 rpm for 10 min to collect blood mononuclear cells.
SNP selection and genotyping
SNPs in the MBL2 gene are associated with variation in quantity and/or function of serum MBL. The polymorphism is determined by the co-occurrence of three promoter variants (H/L, rs11003125; X/Y, rs7096206; and P/Q, rs7095891) and one exon 1 variant (B, rs1800450) (13). DNA from DN patients was sequenced using the sanger two-way sequencing (model 3730; Applied BioSystems). Primer sequences for MBL2 were forward primer (5'-GTTTTCTAATTGCCAGTGG-3') and reverse primer (5'-CAAATAGGACATCAGTCTCC-3').
Statistical analysis
All statistical analyses were performed with SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). The figures were constructed by MedCalc, version 15, or GraphPad Prism 8. The results were expressed as means with standard deviation (SD) for normally distributed continuous variables, median values (interquartile ranges, IQR) for non-normally distributed continuous variables, or frequencies and percentages for categorical variables. The unmeasured results were regarded as deficiency. The patients lost to follow-up were excluded in this retrospective cohort study. Student’s t-test for independent samples was used for comparisons of normally distributed continuous variables. Comparisons of non-normally distributed continuous variables were performed using Mann-Whitney U-test. The Chi-square test was used for categorical variables. The receiver operating characteristic (ROC) curves were used to analyze predictors for ESRD, and the cut-off value was calculated by the maximum of the Youden index (sensitivity + specificity-1). The renal survival was established by Kaplan-Meier curves, and differences in survival rates were assessed with the log-rank test. The risk factors for ESRD were examined via Cox proportional hazards models. Variables statistically significant in univariate analyses entered into multivariate Cox models. A P value <0.05 was considered statistically significant.
Results
The characteristics and MBL levels of DN patients with different renal outcome
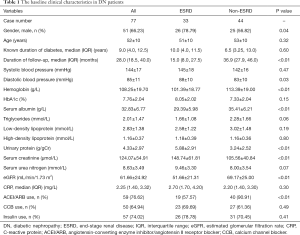
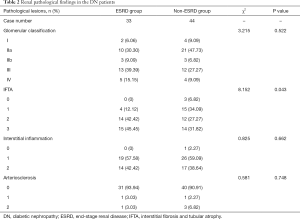
The baseline clinical characteristics of the DN patients were shown in Table 1. Their average age was 52±10 years and their median duration of diabetes history was 9.0 (4.0, 12.5) years. In a median follow-up duration of 28.0 (18.5, 40.0) months after renal biopsy, 33 (42.86%) patients developed ESRD. The patients were divided into ESRD group (33 patients) and non-ESRD group (44 patients). The patients in ESRD group had more males, higher diastolic blood pressure, serum creatinine and urine protein levels, and lower serum albumin, hemoglobin and eGFR levels compared with the patients in non-ESRD group. The patients in ESRD group also had a shorter follow-up duration and lower percentage of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) usage. The renal pathological characteristics in both groups were shown in Table 2. The patients in ESRD group had a higher grade of IFTA than those in non-ESRD group (P<0.05); however, there were no significant differences in glomerular lesions, interstitial inflammation, or arteriosclerosis between the two groups.
Full table
Full table
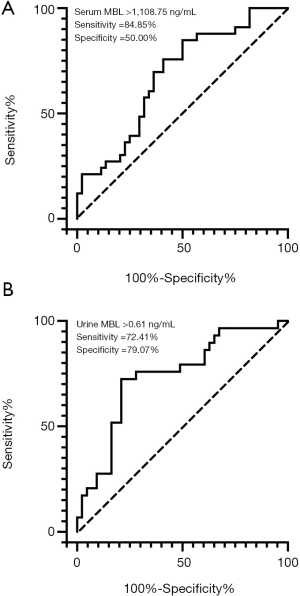
The median (IQR) serum MBL level was substantially higher in ESRD group than that in the non-ESRD group [2,783.75 (1,244.28, 3,837.07) vs. 1,141.60 (652.67, 3,188.44) ng/mL, P=0.016, Figure 2A]. Seventy-two out of the 77 patients had available urine samples. We then measured urine MBL level (normalized for urinary creatinine concentration) and found urine MBL level was also higher in ESRD group than that in non-ESRD group [1.02 (0.43, 2.05) vs. 0.27 (0.04, 0.58) ng/mg, P<0.01, Figure 2B]. The cut-off values based on the maximum value of the Youden index in ROC analysis were serum MBL >1,108.75 ng/mL (Figure 3A) and urine MBL >0.61 ng/mg (Figure 3B) for prediction of ESRD. By univariate Cox analysis, shown in Table 3, serum MBL >1,108.75 ng/mL and urine MBL >0.61 ng/mg were risk factors for ESRD [hazard ratio (HR) =4.164, 95% CI: 1.601–10.833, P=0.003; HR =5.337, 95% CI: 2.332–12.214, P<0.001, respectively]. Adjusted by the characteristics of urine MBL, diastolic blood pressure, hemoglobin, urinary protein, eGFR, and the classification of IFTA, ACEI/ARB usage, serum MBL >1,108.75 ng/mL remained an independent risk factor for ESRD [HR =4.644, 95% CI: 1.320–16.337, P=0.017] by multivariate Cox analysis.


Full table
The SNPs of MBL2 gene in DN patients with different MBL levels
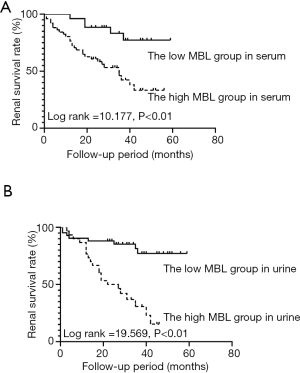
The 77 DN patients were divided low serum MBL group (serum MBL ≤1,108.75 ng/mL) and high serum MBL group (serum MBL >1,108.75 ng/mL) by the cut-off value of serum MBL. The median serum MBL was 542.02 (265.01, 783.02) ng/mL in low serum MBL group, and 3,136.78 (2,023.80, 3,881.09) ng/mL in high serum MBL group (P<0.01). Five (6.84%) patients in low serum MBL group and 28 (38.35%) patients in high serum MBL group reached ESRD during follow-up. Kaplan-Meier survival analysis showed that the renal survival rate was higher in low serum MBL group than that in high serum MBL group (log rank =10.177, P<0.01) (Figure 4A). Also, 72 patients with urine MBL results were divided into low urine MBL group (urine MBL ≤0.61 ng/mg) and high urine MBL group (urine MBL >0.61 ng/mg) by the cut-off value of urine MBL. The median urine MBL level was 0.14 (0.03, 0.37) ng/mg in low urine MBL group, and 1.60 (0.96, 2.28) ng/mg in high urine MBL group (P<0.01). Eight (11.11%) patients in low urine MBL group and 21 (29.16%) patients in high urine MBL group developed ESRD. Kaplan-Meier survival analysis showed that renal survival rate was higher in low urine MBL group than that in the high urine MBL group (log rank =19.569, P<0.01) (Figure 4B).
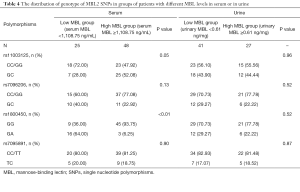
The distribution of the genotype of MBL2 SNPs in groups of patients with different serum or urine MBL levels was shown in Table 4. For SNP rs1800450, the frequencies of homozygous genotype (GG) and heterozygous genotype (GA) were different between high serum MBL group and low serum MBL group (P<0.01); whereas the frequencies were similar between high urine MBL group and low urine MBL group (P=0.52). For SNP rs11003125, rs7096206, and rs7095891, the frequencies were similar in both groups with different serum or urine MBL levels.
Full table
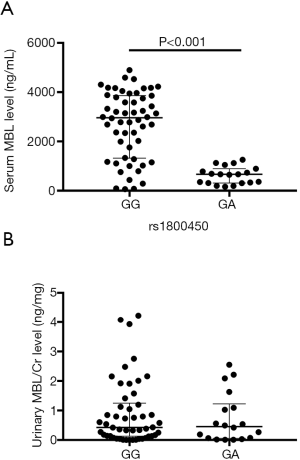
Patients with rs1800450 GG genotype had a higher serum MBL level (median 2,963.52 ng/mL) compared with those with GA genotype (median 665.38 ng/mL) (Z=−5.092, P<0.001) (Figure 5A). However, the urine MBL level was not statistically different between patients with rs1800450 GG genotype and GA genotype (Figure 5B).
The validation of the SNPs in the MBL2 gene in DN patients with different outcomes
In order to validate the correlation of MBL2 SNPs with clinical outcomes, we retrospectively enrolled a new cohort of 133 DN patients with the same inclusion and exclusion criteria as the previous cohort. These patients were admitted in our hospital from March 2005 to March 2018 and SNPs were analyzed. They had no serum or urine samples available. The baseline characteristics of these patients were shown in Tables S1,S2. The MBL2 SNPs of rs7096206, rs11003125, rs1800450, and rs7095891 were shown in Table 5. There were 58 (43.61%) patients in ESRD group and 75 (56.39%) patients in non-ESRD group. The patients in non-ESRD group showed a tendency of higher frequency of rs1800450 GA genotype than that in ESRD group (P=0.053). Furthermore, rs1800450 GA genotype was proven an independent protective factor for ESRD (HR =0.485, 95% CI: 0.237–0.991, P=0.047) by multivariate Cox analysis, adjusted by the characteristics of diastolic blood pressure, hemoglobin, eGFR, and the classification of IFTA, interstitial inflammation and arteriosclerosis, and the MBL2 SNPs of rs11003125, rs7096206, and rs7095891 (Table 6).
Full table

Full table
Discussion
This study showed that the serum and urine MBL levels were higher in DN patients who were prone to develop ESRD, and serum MBL level was an independent risk factor for the progression to ESRD. In addition, the patients with GA genotype of rs1800450 of the MBL2 gene had lower serum MBL level and less possibility of developing ESRD.
Activation of MBL may initiate the regional and systemic inflammation through the cascade of complement and modulation of pro-inflammatory cytokine production (14). Studies showed increased plasma level of MBL was associated with the development of DN in patients with type 2 DM (8,9,15). Also, MBL and MBL-associated serine protease 1 (MASP1) deposited in the renal tubular interstitium, closely correlated with the severity of tubular interstitial injury (8,16). Hansen et al. (17) reported that in type 2 DM patients, measurement of MBL alone or in combination with C-reactive protein (CRP) can provide prognostic information on the development of albuminuria. Another study showed that elevated serum MBL in type 2 DM patients was correlated with the development of DN, especially in combination with serum CRP (18). MBL may also participate in the development of DN through oxidative stress and coagulation cascades (19,20). These studies suggested that MBL may be involved in the mechanism of DN progression. We found that the elevated serum MBL level was an independent risk factor for the progression to ESRD in DN patients, and the risk of ESRD was 4.644 assessed by HR when serum MBL was higher than 1,108.75 ng/mL.
This study investigated the correlation of the MBL2 SNPs with DN progression, and further proved this correlation in a new cohort of DN patients. Bijkerk et al. demonstrated that circulating MBL levels were associated with DN, possibly being dependent on polymorphisms of the MBL2 exon 1 and promoter (21). Similarly, patients with the GA genotype of MBL2 rs1800450 gene had low serum MBL level in our study, consistent with the previous study (13). rs1800450 was the main variant in the exon 1 region of the MBL2 gene and was shown to be associated with the development of type 2 diabetes (22). In Hansen’s study, genotypes with high MBL expression were more frequent in DN patients than those with normal urinary albumin excretion (UAE), and the risk of having nephropathy was 1.52 assessed by odds ratio (OR) given a high MBL genotype (23). Our results indicated that DN patients with heterozygous genotype (GA) of rs1800450 had better renal outcomes than those with homozygous genotype, decreasing the HR of ESRD development in DN patients by 51.5%. These results suggested that the GA genotype of rs1800450 may be valuable for predicting the incidence of ESRD in DN patients. In addition, no correlation was observed between the other gene SNPs and ESRD in the present study, which is consistent with the previous study by Zhang et al. (13)
In conclusion, activation of MBL was correlated with the progression of DN. The rs1800450 SNP of the MBL2 gene may be valued in predicting the incidence of ESRD in DN patients. Considering the limitations of this study as a single-center study with a small sample size, the role of MBL in DN pathogenesis needs to be further confirmed.
Acknowledgments
Funding: This study was supported by the funds from National Key R&D Program of China (2018YFC1314003), and National Natural Science Foundation of China (81570605, 81770674) to Fei Han.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1073
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1073
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1073). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research protocols were conformed to the provisions of the Declaration of Helsinki (as revised in 2013) and were approved by the Ethic Committee of the First Affiliated Hospital of Medical School of Zhejiang University (No. 2017243). All patients were informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Webster AC, Nagler EV, Morton RL, et al. Chronic Kidney Disease. Lancet 2017;389:1238-52. [Crossref] [PubMed]
- Popat RJ, Robson M. Complement and glomerular diseases. Nephron Clin Pract 2014;128:238-42. [Crossref] [PubMed]
- Klessens CQF, Zandbergen M, Wolterbeek R, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant 2017;32:1322-9. [PubMed]
- Moon JY, Jeong KH, Lee TW, et al. Aberrant recruitment and activation of T cells in diabetic nephropathy. Am J Nephrol 2012;35:164-74. [Crossref] [PubMed]
- Nguyen D, Ping F, Mu W, et al. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology 2006;11:226-31. [Crossref] [PubMed]
- Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens 2006;68:193-209. [Crossref] [PubMed]
- Ip WK, Takahashi K, Ezekowitz RA, et al. Mannose-binding lectin and innate immunity. Immunol Rev 2009;230:9-21. [Crossref] [PubMed]
- Zheng JM, Ren XG, Jiang ZH, et al. Lectin-induced renal local complement activation is involved in tubular interstitial injury in diabetic nephropathy. Clin Chim Acta 2018;482:65-73. [Crossref] [PubMed]
- Li XQ, Chang DY, Chen M, et al. Complement activation in patients with diabetic nephropathy. Diabetes Metab 2019;45:248-53. [Crossref] [PubMed]
- Bus P, Chua JS, Klessens CQF, et al. Complement Activation in Patients With Diabetic Nephropathy. Kidney Int Rep 2017;3:302-13. [Crossref] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin Diabetes 2017;35:5-26. [PubMed]
- Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010;21:556-63. [Crossref] [PubMed]
- Zhang N, Zhuang M, Ma A, et al. Association of levels of mannose-binding lectin and the MBL2 gene with type 2 diabetes and diabetic nephropathy. PLoS One 2013;8:e83059. [Crossref] [PubMed]
- Collard CD, Väkevä A, Morrissey MA, et al. Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol 2000;156:1549-56. [Crossref] [PubMed]
- Guan LZ, Tong Q, Xu J. Elevated serum levels of mannose-binding lectin and diabetic nephropathy in type 2 diabetes. PLoS One 2015;10:e0119699. [Crossref] [PubMed]
- Huang Y, Xu J, Wu X, et al. High Expression of Complement Components in the Kidneys of Type 2 Diabetic Rats with Diabetic Nephropathy. Front Endocrinol (Lausanne) 2019;10:459. [Crossref] [PubMed]
- Hansen TK, Gall MA, Tarnow L, et al. Mannose-binding lectin and mortality in type 2 diabetes. Arch Intern Med 2006;166:2007-13. [Crossref] [PubMed]
- Elawa G. The predictive value of serum mannan-binding lectin levels for diabetic control and renal complications in type 2 diabetic patients. Saudi Med J 2011;32:784-90. [PubMed]
- Pan HZ, Zhang L, Guo MY, et al. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol 2010.71-6. [Crossref] [PubMed]
- Aso Y, Yoshida N, Okumura K, et al. Coagulation and inflammation in overt diabetic nephropathy: association with hyperhomocysteinemia. Clin Chim Acta 2004;348:139-45. [Crossref] [PubMed]
- Bijkerk R, van der Pol P, Khairoun M, et al. Simultaneous pancreas-kidney transplantation in patients with type 1 diabetes reverses elevated MBL levels in association with MBL2 genotype and VEGF expression. Diabetologia 2016;59:853-8. [Crossref] [PubMed]
- Muller YL, Hanson RL, Bian L, et al. Functional variants in MBL2 are associated with type 2 diabetes and pre-diabetes traits in Pima Indians and the old order Amish. Diabetes 2010;59:2080-5. [Crossref] [PubMed]
- Mellbin LG, Hamsten A, Malmberg K, et al. Mannose-binding lectin genotype and phenotype in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care 2010;33:2451-6. [Crossref] [PubMed]