
Hemovac blood after total knee arthroplasty as a source of stem cells
Introduction
Active and continuous efforts have been undertaken in various fields to improve the quality of life as life expectancy has risen significantly over the years. Especially in the medical field, stem cell research is actively progressing in an effort to overcome intractable diseases and maintain quality health during an individual’s lifetime (1,2). For stem cell research, diverse tissues, such as umbilical cord blood, bone marrow (3), adipose tissue (4), muscle, teeth (5), cartilage, and synovium are being used as stem cell sources (6-8). Although stem cells from animal tissues can be used for research, their importance and value are lower than those from human tissues. Recently, due to ethical misconduct, reckless stem cell research has been prohibited, and strict regulatory guidelines have been reinforced by the Institutional Review Boards (IRBs) to protect patient rights. Therefore, it would not be possible to receive approval from an IRB or ethics committee if the research is found to be counterintuitive to the patients’ interests.
Total knee arthroplasty (TKA) is the gold standard for end-stage osteoarthritis, and a large number of TKA surgeries are performed each year (9,10). Blood collects inside the joint after surgery, and hence, a hemovac line is placed inside the joint post-surgery to decompress the joint, controlling pain and swelling (11-13). A hemovac drain can collect a sufficient quantity of blood to provide adequate numbers of cells. Since the blood is from both the damaged tissue and the bone cutting site, the bone marrow components are included in the hemovac blood (HVB). During a TKA surgery, cutting the surface of cancellous bones including the distal femur or proximal tibia often results in bone marrow leaking from the cut surface. Within the bone marrow aspirate (BMA), a fair amount of venous blood is also often included. Since the stromal component of bone marrow can be used to treat musculoskeletal diseases, such as nonunion fractures or cartilage defects, centrifugation and concentration of the stromal component is often performed to allow for effective clinical treatment. Similarly, HVB can also include bone marrow components; however, the amount of stromal blood is often much less than what is observed in BMAs. As such, a concentration procedure is often necessary. Hence, this study was undertaken to investigate if HVB can be manipulated in such a way to render it a useful source of stem cells for research.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/atm-20-2215).
Methods
Patients
The HVB of 20 patients who underwent TKA was used for this study. The average age of the patients was 72.9 years [range, 57–86 years; standard deviation (SD), 7.56]. BMAs from 15 patients who underwent the arthroscopic cartilage repair procedure were concentrated, and the samples remaining after the procedure were used for comparative analysis. The average age of these patients was 72.1 years (range, 61–80 years; SD, 5.18). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (UC18DESI0056) and informed consent was obtained from each patient.
Bone marrow aspiration and concentration
Bone marrow aspirate concentrate (BMAC) was obtained by inserting a bone marrow aspiration needle (SPASY™, Seoul, Korea) into the anterior superior iliac spine after sterilization under general anesthesia. Aspiration was then performed using a 50-mL syringe, including 4 mL of the anticoagulant citrate dextrose solution (Huons ACD Injection, Huons, Seongnam, Korea), after which 40 mL of BMA, including 4 mL of ACD solution, was transferred to a BMC kit (Revmed, Seongnam, Korea). Two cycles of centrifugation were performed to obtain the BMAC. The first cycle was for 6 min at 2,700 ×g, followed by a second cycle for 5 min at 2,400 ×g (Figure 1).

HVB aspiration and concentration
Blood (40 mL), including 4 mL of ACD solution, was aspirated from the hemovac immediately after TKA surgery. The aspirated blood was transferred to the kit (BMC kit™, Revmed, Seongnam, Korea), and two cycles of centrifugation were performed to obtain the HVB concentrate (HVBC) (Figure 1).
BMAC and HVBC analysis
Cell counts
The numbers of nucleated cells, lymphocytes, monocytes, red blood cells, platelets, and neutrophil granulocytes in the BMA, BMAC, HVB, and HVBC were counted using a cell counter (XE2100, Sysmex, Japan). The number of mononuclear cells (MNCs) was calculated by summing the number of lymphocytes and monocytes.
Isolation of BMA and HVB MNCs
BMAs were mixed with PBS (Gibco Invitrogen, Grand Island, NY, USA) at a 1:1 ratio. The mixture was loaded onto a histopaque layer (1.077 g/mL; Sigma chemical co., St. Louis, MO, USA). MNCs were separated by density gradient centrifugation (400 ×g, 25 min, room temperature), washed thrice with alpha minimum essential medium (αMEM; Gibco Invitrogen, Grand Island, NY, USA), filtered using a 70-µm cell strainer (Becton Dickinson, Falcon, Germany), and resuspended. The cells were incubated at 37 °C with 5% CO2 in basic medium (αMEM containing 10% FBS, 100 units/mL penicillin, 100 µg/mL streptomycin). The culture flask was washed with PBS to remove the non-adherent cells and incubated further until adherent cells reached confluence. The confluent cells were trypsinized (0.25% trypsin EDTA), divided into several culture flasks, and incubated in the basic medium. This subculture was performed for further studies, and the same procedure was repeated for HVB.
Colony forming unit-fibroblast (CFU-F) assay
A CFU-F assay was performed to quantify non-hematopoietic fibroblastic colonies. The isolated mononucleated cells were cultured in 60-mm cell culture dishes (Becton Dickinson/Falcon, Germany) for 14 days at 37 °C in a humidified atmosphere and 5% CO2. αMEM (Gibco, Life Technologies, Karlsruhe, Germany) medium supplemented with 20% FBS (Gibco), penicillin (100 U/mL; Gibco), and streptomycin (100 µg/mL; Gibco) was used for culturing.
After 14 days, adherent cells were fixed with 4% paraformaldehyde (Biosesang, Seongnam, Korea) for 5 min, washed with PBS, and stained with 1% crystal violet. The culture plate was placed on a white paper, and colonies were counted macroscopically. The number of stained cell colonies larger than 2.5 mm in diameter was counted (14).
Flow cytometry
Surface marker analysis of HVB, HVBC, BMA, and BMAC was performed using a FACSCalibur flow cytometer (BD Biosciences, Heidelberg, Germany). The harvested cells were fixed in 4% paraformaldehyde at 4 °C for 30 min and washed in flow cytometry buffer (FCB, BD Biosciences, Heidelberg, Germany). Subsequently, the cells were incubated with a blocking buffer for 30 min, followed by centrifugation to remove the blocking buffer. The cells were aliquoted at 1×106 for antibody treatment. Cells were stained with PE-labeled antibodies (Abs; CD44, CD29) and APC-labeled Abs (CD90, CD105) (BD Biosciences, San Jose, CA, USA).
Cell proliferation assay
Cell proliferation was assessed using the cell counting kit (CCK-8) assay (Dojindo, Japan). Briefly, mesenchymal stem cells (MSCs) isolated from the HVBC and BMAC were seeded at 1×105 cells/well in a 96-well plate. The assay was performed from 1 to 7 days after cell seeding. MSCs from each time point were mixed with 10 uL of CCK-8 solution/well and incubated for 2 h at 37 °C. The cellular dehydrogenase activity of the cells was then measured at 450 nm using a microplate reader. The assay was performed in triplicate.
Transwell migration assay
Migration assays were performed in Transwell plates (cat no. 3422, Corning Costar, Cambridge, MA, USA), 6.5 mm in diameter with 8 µm pore filters. P2 MSCs (5×105 cells) isolated from the HVBC and BMAC were added to the upper chamber in basal medium (αMEM without FBS). After overnight culture, 20% FBS αMEM was added to the bottom chamber. Basal medium served as a negative control. After 12 h incubation at 37 °C, with 5% CO2, the upper chamber of the filters was carefully washed with cold PBS, and cells remaining on the upper side were removed with a cotton swab. Migrated cells through the chamber, or adhered to the lower membrane, were fix in 4% paraformaldehyde. After staining with 0.1% crystal violet, the images were observed microscopically. The absorbance of migrated cells was measured at 520 nm after being dissolved in 100% methanol. Each experiment was performed in triplicate.
Multi-lineage differentiation
MSCs isolated from the HVBC and BMAC were tested for their osteogenic, adipogenic, and chondrogenic differentiation potentials. Briefly, the cells (2×104 cells/well, passage 3) were seeded in 6-well plates and treated with the osteogenic and adipogenic induction medium for 2 weeks. Culture media were changed every 3 days. For osteogenic differentiation, cultured cells were washed thrice with DPBS (Gibco Invitrogen, Grand Island, NY, USA) and fixed in 4% paraformaldehyde at 4 °C for 10 min. Mineralization of the extracellular matrix as an indicator of osteogenic differentiation was observed using Alizarin red S and alkaline phosphatase (ALP) staining (15,16).
For adipogenic differentiation, cells were washed thrice with DPBS and fixed in 4% paraformaldehyde at 4 °C for 20 min. The cells were treated with Oil Red O staining dye to visualize the lipids. For quantitative measurements, Oil Red O was eluted by dissolving in isopropanol, and the absorbance was measured at 520 nm.
For chondrogenic differentiation, the pelleted culture was used. Here, 2.5×105 cells (passage 3) were resuspended in the chondrogenic differentiation medium in 15-mL polypropylene tubes. After the cells were centrifuged at 500 ×g for 5 min to form aggregates, they were incubated at 37 °C in a humidified atmosphere of 5% CO2. The aggregates were cultivated for 21 days, and the medium was changed every 3 days. On the 21st day, the cell pellets were fixed in 10% formalin and embedded in paraffin. Sections of the cell pellets were stained with type II collagen, Alcian blue (pH 2.5), Safranin O, and toluidine blue to identify the collagen content and sulfated proteoglycans in the extracellular matrix. Hematoxylin-eosin staining was performed for morphological observation of the cells.
Adipogenic differentiation medium
This medium was comprised of the following: Dulbecco’s modified Eagle’s medium-high glucose (DMEM-HG; 11965-084, Gibco-Life Technologies, Carlsbad, CA, USA) containing 10−6 M dexamethasone, 10 µg/mL insulin, 100 µM indomethacin, and 500 µM 3-isobutyl-1-methylxanthine.
Osteogenic differentiation medium
This medium was comprised of the following: αMEM (12571-063, Gibco-Life Technologies, Carlsbad, CA, USA) containing 50 µg/mL L-ascorbic acid, 10 mM b-glycerophosphate, and 10 nM dexamethasone.
Chondrogenic differentiation medium
This medium was comprised of the following: DMEM-HG (11965-084, Gibco-Life Technologies, Carlsbad, CA, USA) containing 10−7 M dexamethasone, 10 ng/mL transforming growth factor beta 3 (TGF-β3), 100 µg/mL sodium pyruvate, 40 µg/mL proline, 25 µM ascorbic acid-2-phosphate, 100 U/mL penicillin, 100 µg/mL streptomycin, and 1% (v/v) ITS plus (5 µg/mL insulin, 5 µg/mL transferrin, 5 µg/mL selenous acid). All reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from the MSCs of HVBC and BMAC showing osteogenic, adipogenic, and chondrogenic differentiation was extracted using the RNeasy mini kit (74104, Qiagen, Hilden, Germany). The RNA samples were reverse transcribed into cDNA using the QuantiTect reverse transcription kit (205311, Qiagen, Hilden, Germany) following the manufacturer’s instruction. All samples were analyzed using SYBR green (A6001, Promega, Madison, WI, USA) on a Promega qPCR system, and the relative expression levels were determined according to the 2−∆∆Ct method. Primer sequences used for the qRT-PCR analysis is provided in Table 1.
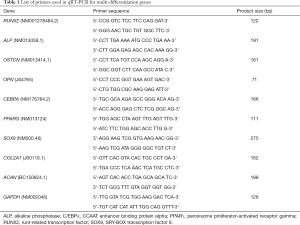
Full table
Statistical analysis
Statistical analysis was performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). All variables were summarized using standard descriptive statistics such as mean, SD, median, and range. The Mann-Whitney test was used for comparative analyses between groups. Statistical significance is described as P<0.05.
Results
Automatic cell counts
Automatic cell counting was performed to compare the number of cells before and after concentrating BMA and HVB. The cells were increased in BMAC and HVBC (Table 2).
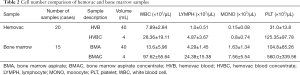
Full table
Colony forming unit-fibroblast assay
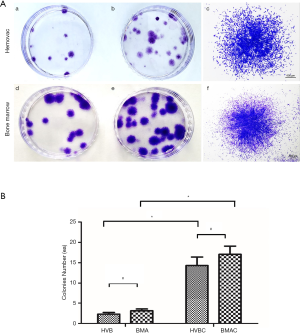
CFU-F assay results demonstrate the self-renewal capacity of cells able to form new fibroblast colonies from single cells. The density and size of the colonies were found to be different according to the proliferative status of cells. A higher density and larger size of colonies indicates the higher proliferative potential of cells (14). Small fibroblastic colonies were observed on culture plates isolated from HVB and BMA at 5–7 days of culture. After 14 days, colonies were analyzed using a light microscope with crystal violet staining. The number of colonies that were >2.5 mm in size was counted (Figure 2A). The number of colonies were 2.30±1.87 (20 patients) in HVB, and 3.13±1.73 (15 patients) in BMA, with no statistically significant difference (P=0.365). Meanwhile, there were 14.30±9.45 (20 patients) colonies in HVBC, and 17.07±7.89 (15 patients) in BMAC, without statistically significant differences observed (P=0.187). However, the number of colonies obtained before and after concentration were significantly different in each group (P<0.05; Figure 2B).
Flow cytometry
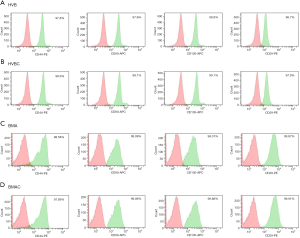
Surface expression markers in HVB, HVBC, BMA, and BMAC were confirmed by flow cytometry. CD29, CD44, CD90, and CD105, which are MSC-specific markers, were evaluated. All were highly expressed (>96%) in HVB, HVBC, BMA, and BMAC (Figure 3). However, CD34 (0.18% in HVB and 0.39% in HVBC, 0.08% in BMA and 2.82% in BMAC), CD45 (0.41% in HVB and 0.49% in HVBC, 0.28% in BMA and 3.08% in BMAC), and HLA-DR (0.21% in HVB and 0.12% in HVB, 0.98% in BMA and 0.18% in BMAC) showed negative expression (<4%). Figure 3 shows a summary of the surface marker expression analysis of HVB, HVBC, BMA, and BMAC, which was associated with no significant variation.
Isolated cells from HVB and BMA
Cells isolated from HVB and BMA were cultured and observed 3–5 days after the initial plating Figure 4A (a, c), and maintained in αMEM supplemented with 10% FBS and penicillin. These cells readily expanded in vitro, attached to the well, and showed a fibroblast-like morphology. The viability of the cells in each passage was greater than 98%, with no morphological changes observed Figure 4A (b, d).
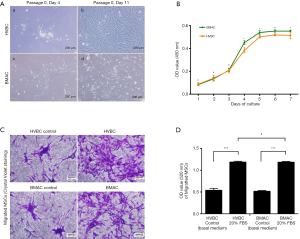
Cell proliferation
The proliferation of MSCs isolated from the HVBC and BMAC was observed. After day 1 to 7 of culturing, the optical density (OD) of cells increased from 0.087±0.012 to 0.508±0.028 in HVBC, and 0.083±0.010 to 0.552±0.003 in BMAC (average ± SD; Figure 4B). There was no significant difference between the HVBC and BMAC in OD value from days 1 to 3 (Figure 4B, P<0.05). Meanwhile, the OD values representing proliferation during days 4 to 7 were significantly different between the HVBC and BMAC (Figure 4B, P<0.05, P<0.01). The OD values were 0.383±0.022 on day 4, 0.502±0.011 on day 5, 0.519±0.009 on day 6 in HVBC, and 0.451±0.016 on day 4, 0.540±0.016 on day 5, and 0.553±0.004 on day 6 in BMAC. The pattern of increasing OD values for cell numbers between the HVBC and BMAC was similar.
Cell migration
Next, the migration ability of MSCs isolated from the HVBC and BMAC was observed using crystal violet staining. The cells from the HVBC and BMAC were morphologically similar (Figure 4C). The measurement of the OD value of stained cells represents the number of migrated cells. The OD value of 20% FBS αMEM was 1.182±0.024, which was approximately 2.2-fold higher than the control (αMEM without FBS; 0.538±0.045) in HVBC. Meanwhile, the OD value of 20% FBS αMEM was 1.18±0.019, and approximately 2.3-fold higher than the control (αMEM without FBS; 0.515±0.019) in BMAC. There was no significant difference between the HVBC and BMAC in OD values (Figure 4D, P<0.05).
Multi-lineage differentiation (chondrogenic, osteogenic, and adipogenic)
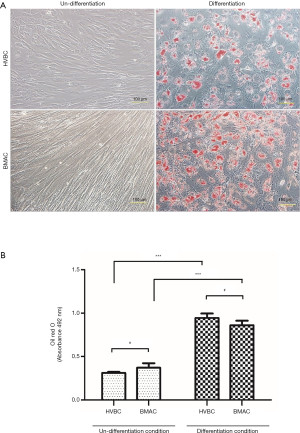
Adipogenic differentiation was confirmed by Oil Red O staining to identify intracellular lipids. Intracellular lipid droplets in isolated cells from HVB and BMA were observed. Lipid droplets were not found in the undifferentiated condition (Figure 5A). Absorbance values were 0.311±0.011 nm for HVBC, and 0.371±0.051 nm for BMAC in the undifferentiated condition, and 0.945±0.051 nm for HVBC, and 0.860±0.051 nm for BMAC in the differentiated condition (Figure 5B). Absorbance values in the undifferentiated and differentiated conditions between HVBC and BMAC were not significantly different (P>0.05). However, absorbance between the undifferentiated and differentiated conditions for HVBC and BMAC were significant (P<0.05; Figure 5B).
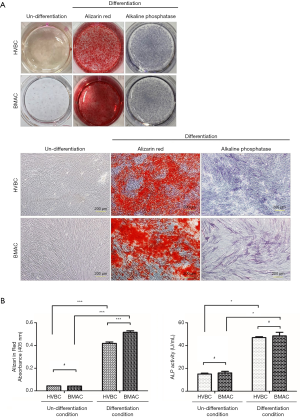
Osteogenic differentiation was identified by Alizarin red S (calcification of the extracellular matrix) and ALP staining (Figure 6). Calcification during osteogenic differentiation appears as large crystal clusters among cells. Crystal clusters (calcification in the extracellular matrix) were stained red with Alizarin red S staining (Figure 6A). Absorbance values were 0.044±0.001 nm for HVBC and 0.042±0.001 nm for BMAC in the undifferentiated condition and 0.419±0.012 nm for HVBC and 0.516±0.013 nm for BMAC in the differentiated condition. Absorbance values between HVBC and BMAC in the differentiated condition were significantly different after Alizarin red staining (P<0.001) with a higher value in BMAC (Figure 6B). The absorbance value of the undifferentiated and differentiated conditions between HVBC and BMAC in the ALP assay did not show any significant difference with values of 47.025±0.837 U/mL in HVBC, and 48.548±3.195 U/mL in BMAC (P>0.05; Figure 6B).
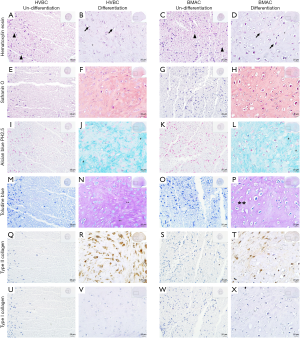
To evaluate chondrogenic differentiation, hematoxylin-eosin staining was performed to observe the morphology of cells, and Alcian blue pH (2.5) and toluidine blue staining was performed to observe the pericellular proteoglycan and type II collagen deposition (Figure 7). Many round cells with surrounding lacuna were observed after hematoxylin-eosin staining (black arrow, Figure 7B,D). The rich extracellular matrix surrounding the cells was observed in the chondrogenic differentiation group of both HVBC and BMAC (Figure 7B,D). The pellets in the differentiated condition showed strong toluidine blue (Figure 7N,P) and Alcian blue pH (2.5) staining (indicative of cartilage matrix; # Figure 7J,L). Safranin O staining and the morphology of the cells suggested that a cartilaginous matrix had been synthesized. The highly stained area after toluidine blue and Alcian blue pH (2.5) staining corresponded to the safranin O-stained areas in the chondrogenic differentiation condition (Figure 7F,H). Type II collagen expression inn HVBC and BMAC was confirmed via immunohistochemical staining (Figure 7R,T). Type II collagen is the main collagen component of the extracellular matrix and comprises the cartilage-specific matrix (17,18). Type I collagen was not detected in any of the groups (Figure 7V,X).
Gene expression
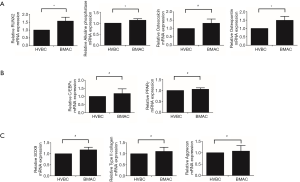
Total RNA was extracted from MSCs in HVBC and BMAC on the 14th day of culture after osteogenic and adipogenic differentiation, and on the 21st day of chondrogenic differentiation. Expression of the genes for runt-related transcription factor 2 (RUNX2), osteopontin (OPN), osteocalcin (OSTCN), and ALP, were analyzed as osteogenic differentiation markers. RUNX2, OPN and ALP expression levels were higher in BMAC than in HVBC. However, OSTCN expression levels in HVBC and BMAC were not significantly different (Figure 8A; P<0.05, P>0.05). Further, the adipogenic differentiation markers PPARG and CEBPA were observed. The expression of PPARG and CEBPA genes after adipogenic differentiation was upregulated; however, the difference between HVBC and BMAC was not significant (Figure 8B, P>0.05). Additionally, the genes for type II collagen (COLA1), aggrecan (ACAN), and SOX9 were investigated as chondrogenic differentiation-specific markers (Figure 8C). The expression of all three was significantly upregulated in the chondrogenic differentiation group; however, the difference between HVBC and BMAC was not statistically significant (Figure 8C, P>0.05).
Discussion
Since stem cell research is an actively progressing field, finding an adequate cell source is critical. Bone (19), bone marrow (20), synovium (21,22), adipose tissue (23), cord blood (24), are well known sources (25-27). Additionally, the nasal septum, muscle, and cartilage serve as stem cell sources for musculoskeletal regeneration (28-31).
Bone marrow mesenchymal stem cells (BMSCs) have served as the primary source of stem cells for many years. However, harvesting BMSCs is a painful procedure and they exhibit signs of senescence at an early stage of expansion compared with MSCs derived from other sources (32,33). Nevertheless, BMSCs require a relatively short culture period (34,35), and many clinical studies using this source are actively being conducted. Alternatively, adipose tissue derived MSCs can be readily isolated (35) with morphological and phenotypical characteristics similar to BMSCs, which are stable during the culture period (36). Moreover, although umbilical cord blood derived MSCs (UCB-MSCs) have an associated long cultivation period, they exhibit high proliferation capacity (37,38). Meanwhile, peripheral blood derived MSCs are easily obtained, which is the reason for their application in many animal studies (38-45), however, they are present at low levels in mononuclear cells (38).
Compared to the other sources of stem cells, HVB can be readily obtained after various bone surgeries, including TKA, without the need for special procedures that can cause additional pain and be met with ethical issues. Although the amount of HVB may vary between patients, a sufficient number of MSCs can be obtained through concentration and cultivation of the cells. Nevertheless, the hemovac must be treated carefully and aseptically following TKA. Should the HVB become contaminated, the hemovac must be removed immediately from the patient to avoid the complications associated with TKA infection. Hence, to be used as a regular source of stem cells, an advanced care protocol for hemovac is required.
TKA is a widely performed surgery and considered to be the gold standard for treatment of late-stage osteoarthritis (9,10). During this surgical procedure, the degenerated bone and cartilage are cut, thereby releasing a large amount of bone marrow components from the bone cutting surface. After the operation, the hemovac line can be inserted intra-articularly to decompress the knee joint thereby preventing hematoma formation. The blood collected in the hemovac contains bone marrow components, which can be used for stem cell research. Most importantly, HVB can be obtained without causing additional pain or harm to the patient. Through the centrifugation of HVB, a sufficient number of cells that have multi-lineage differentiation capacity as stem cells can be obtained. Although the number of cells from HVB and HVBC was very low compared to that from BMA and BMAC, the number of colonies from HVBC and BMAC was not significantly different (#, P>0.05). These cells are actual participants in tissue regeneration.
The cells derived from HVB adhered to the culture flask with fibroblast-like morphology, showed multi-lineage differentiation, and expressed stem cell markers (46). CD marker analysis of HVB and HVBC showed over 96% expression of positive markers (CD29, CD44, CD90, CD105) of MSCs. These results were very similar to those obtained with BMA and BMAC and showed no significant differences.
The multi-differentiation potentials of HVB were evaluated through the culture of cells derived from HVB in osteogenic, adipogenic, and chondrogenic induction medium (46). Alizarin red S (calcium deposit, mineralization) and ALP activity were used for osteogenic differentiation evaluation; BMA showed a higher level of Alizarin red staining, indicating calcium deposit and mineralization. However, the difference in ALP activity between HVB and BMA was not significant. Higher gene expression in BMA was observed for RUNX2, ALP, and osteopontin, but not for osteocalcin.
Peroxisome proliferator-activated receptor-γ (PPARγ) plays an important role in adipogenic differentiation (47). Lipid drop formation was observed using Oil Red O staining, and PPARγ and C/EBPα, as adipocyte differentiation markers, were expressed in HVB and HVBC. The Alcian blue (pH 2.5), safranin O, toluidine blue, and type II collagen staining of cells in HVB and HVBC showed chondrogenic differentiation in the form of characteristic matrix synthesis and the formation of cells with surrounding lacuna. Additionally, specific chondrogenesis markers (1) of gene expression, such as type II collagen, SOX9, and aggrecan, were upregulated after 21 days of culture in chondrogenic conditions. The expression of SOX9, aggrecan, and type II collagen did not show any statistically significant difference between HVB and HVBC.
The stem cells from HVB showed characteristic multi-potentiality, and a sufficient number of cells could be obtained through centrifugation without any harm or pain to the patients. Through this study, we comprehensively demonstrated that the stem cells from HVB could be potentially used in stem cell research involving musculoskeletal regeneration, including that of cartilage, bone, and fat. Moreover, these stem cells can also be explored for their clinical application potential.
After undergoing a TKA operation, patients return to their beds from the surgical theater with a hemovac placed in the knee joint. If the hemovac becomes full of blood, the contents are discarded and new blood begins to fill the hemovac. These first two hemovacs serve as potential sources of stem cells, as after this, the majority of the content will consist of venous blood. The hemovac can hold up to 400 mL of blood, and the kit that was used in this study processes 40 mL. Hence, theoretically, 800 mL can be collected from the first and second hemovac, which can be subsequently concentrated using 20 kits. Each kit can produce 4 mL of HVBC with counts obtained as follows: white blood cells, 28.36±19.11×103/µL; lymphocytes, 4.87±3.67×103/µL; monocytes, 0.8±0.74×103/µL; platelets, 125.35±97.78×103/µL (Table 2). It is, therefore, possible to ultimately collect 20 times more than each of these cell counts from a single patient with this method.
HVBC can produce modified autologous stem cells that can be stored for future clinical application. Moreover, if necessary, cells can be cultured to produce enough cells for clinical applications. Therefore, this method might prove to be practical and efficient for clinical trials. For example, if patients suffer from nonunion fractures, osteonecrosis of the femoral head, or osteoarthritis of the knee joint, we can use these banked stem cells as therapeutic options. However, for this kind of clinical application, more laboratory studies are required and clinical trials must be performed.
Recently, studies have been reported on the injection of BMAC into knee joints (48-53). However, bone marrow aspiration must be performed in the operation theater under aseptic conditions with adequate anesthesia according to patient’s condition. Further, this procedure is also painful. If HVBC from unilateral TKA can be stored, the cells from this source can be injected into the contralateral knee joints to improve the patient’s arthritis-related symptoms.
In this study, we also investigated the optimal time for HVB harvesting. Theoretically, that obtained immediately after surgery is considered of higher quality than that collected later. However, more stem cells were observed in the second HVB aspiration than in the first. There is a possibility that wound irrigation with normal saline prior to completing the operation might dilute the cells from the bone marrow.
To the best of our knowledge, there has been no previous report regarding MSCs derived from HVB. This study demonstrated the morphology, proliferative potential, surface markers, and multi-differentiation ability of the cells obtained from this source, and every aspect was found to be comparable to that of bone marrow-derived stem cells. In conclusion, HVB obtained after total knee replacement can be used as a source of stem cell research or autologous stem cell therapy without the need for invasive and painful bone marrow aspiration procedures.
Acknowledgments
Funding: This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) [NRF-2017M3A9B4028022].
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/atm-20-2215
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2215
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-2215
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2215). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (UC18DESI0056) and informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giri TK, Alexander A, Agrawal M, et al. Current status of stem cell therapies in tissue repair and regeneration. Curr Stem Cell Res Ther 2019;14:117-26. [Crossref] [PubMed]
- Zakrzewski W, Dobrzyński M, Szymonowicz M, et al. Stem cells: past, present, and future. Stem Cell Res Ther 2019;10:68. [Crossref] [PubMed]
- Méndez‐Ferrer S, Scadden DT, Sánchez‐Aguilera A. Bone marrow stem cells: current and emerging concepts. Ann N Y Acad Sci 2015;1335:32-44. [Crossref] [PubMed]
- Wankhade UD, Shen M, Kolhe R, et al. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int 2016;2016:3206807. [Crossref] [PubMed]
- Baniebrahimi G, Khanmohammadi R, Mir F. Teeth‐derived stem cells: A source for cell therapy. J Cell Physiol 2019;234:2426-35. [Crossref] [PubMed]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007;213:341-7. [Crossref] [PubMed]
- Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev 2008;4:3-11. [Crossref] [PubMed]
- De Luca M, Aiuti A, Cossu G, et al. Advances in stem cell research and therapeutic development. Nat Cell Biol 2019;21:801-11. [Crossref] [PubMed]
- Zeni JA, Axe MJ, Snyder-Mackler L. Clinical predictors of elective total joint replacement in persons with end-stage knee osteoarthritis. BMC Musculoskelet Disord 2010;11:86. [Crossref] [PubMed]
- Baier C, Lüring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci 2013;18:955-61. [Crossref] [PubMed]
- Jaafar S, Vigdorchik J, Markel DC. Drain technique in elective total joint arthroplasty. Orthopedics 2014;37:37-9. [Crossref] [PubMed]
- Basaran H, Dasar U, Satılmıs B, et al. Usage of Positive pressure Hemovac Drain following Total Knee Arthroplasty: Reduce Blood Lossor Not. SM J Orthop 2016;2:1027.
- Ayub K, Patterson D, Irani S, et al. Endoscopic ultrasound directed pseudocyst drainage without the use of fluoroscopy: a case series. Gastrointest Endosc 2009;69:S234-S.
- Gothard D, Dawson J, Oreffo R. Assessing the potential of colony morphology for dissecting the CFU-F population from human bone marrow stromal cells. Cell Tissue Res 2013;352:237-47. [Crossref] [PubMed]
- Puchtler H, Meloan SN, Terry MS. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem 1969;17:110-24. [Crossref] [PubMed]
- Hoemann C, El-Gabalawy H, McKee M. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathologie Biologie 2009;57:318-23. [Crossref] [PubMed]
- Little CJ, Bawolin NK, Chen X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng Part B Rev 2011;17:213-27. [Crossref] [PubMed]
- Kuhne M, John T, El-Sayed K, et al. Characterization of auricular chondrocytes and auricular/articular chondrocyte co-cultures in terms of an application in articular cartilage repair. Int J Mol Med 2010;25:701-8. [PubMed]
- Mohsin S, Houser SR. Cortical Bone Derived Stem Cells for Cardiac Wound Healing. Korean Circ J 2019;49:314-25. [Crossref] [PubMed]
- Jin H, Bae Y, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci 2013;14:17986-8001. [Crossref] [PubMed]
- Khan WS, To K, Zhang B, et al. Synovium-Derived Mesenchymal Stem Cell Transplantation in Cartilage Regeneration: A PRISMA Review of in vivo Studies. Front Bioeng Biotechnol 2019;7:314. [Crossref] [PubMed]
- Zupan J, Drobnič M, Stražar K. Synovium-Derived Mesenchymal Stem/Stromal Cells and their Promise for Cartilage Regeneration. Adv Exp Med Biol 2020;1212:87-106. [Crossref] [PubMed]
- Si Z, Wang X, Sun C, et al. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed Pharmacother 2019;114:108765. [Crossref] [PubMed]
- Hoffmann A, Floerkemeier T, Melzer C, et al. Comparison of in vitro‐cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J Tissue Eng Regen Med 2017;11:2565-81. [Crossref] [PubMed]
- Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells 2019;8:886. [Crossref] [PubMed]
- Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig 2019;6:19. [Crossref] [PubMed]
- Trohatou O, Roubelakis MG. Mesenchymal stem/stromal cells in regenerative medicine: past, present, and future. Cell Reprogram 2017;19:217-24. [Crossref] [PubMed]
- Rothrauff BB, Pirosa A, Lin H, et al. Stem Cell Therapy for Musculoskeletal Diseases. Available online: https://www.sciencedirect.com/science/article/pii/B9780128098806000540
- Piuzzi NS, Ng M, Chughtai M, et al. Accelerated Growth of Cellular Therapy Trials in Musculoskeletal Disorders: An Analysis of the NIH Clinical Trials Data Bank. c [Crossref] [PubMed]
- Robinson PG, Murray IR, West CC, et al. Reporting of Mesenchymal Stem Cell Preparation Protocols and Composition: A Systematic Review of the Clinical Orthopaedic Literature. Am J Sports Med 2019;47:991-1000. [Crossref] [PubMed]
- Angadi DS, Macdonald H, Atwal N. Autologous cell-free serum preparations in the management of knee osteoarthritis: what is the current clinical evidence? Knee Surg Relat Res 2020;32:16. [Crossref] [PubMed]
- Berebichez-Fridman R, Gómez-García R, Granados-Montiel J, et al. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells-Their Current Uses and Potential Applications. Stem Cells Int 2017;2017:2638305. [Crossref] [PubMed]
- Cheng HY, Ghetu N, Wallace C, et al. The impact of mesenchymal stem cell source on proliferation, differentiation, immunomodulation and therapeutic efficacy. J Stem Cell Res Ther 2014;4:1-8.
- Cagliani J, Grande D, Molmenti EP, et al. Immunomodulation by mesenchymal stromal cells and their clinical applications. J Stem Cell Regen Biol 2017;3. [Crossref] [PubMed]
- Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294-301. [Crossref] [PubMed]
- Choudhery MS, Badowski M, Muise A, et al. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med 2014;12:8. [Crossref] [PubMed]
- Gang EJ, Hong SH, Jeong JA, et al. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun 2004;321:102-8. [Crossref] [PubMed]
- Lotfy A, El-Sherbiny YM, Cuthbert R, et al. Comparative study of biological characteristics of mesenchymal stem cells isolated from mouse bone marrow and peripheral blood. Biomed Rep 2019;11:165-70. [Crossref] [PubMed]
- He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells 2007;25:69-77. [Crossref] [PubMed]
- Koerner J, Nesic D, Romero JD, et al. Equine peripheral blood‐derived progenitors in comparison to bone marrow‐derived mesenchymal stem cells. Stem Cells 2006;24:1613-9. [Crossref] [PubMed]
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2000;2:477-88. [Crossref] [PubMed]
- Fu WL, Zhang JY, Fu X, et al. Comparative study of the biological characteristics of mesenchymal stem cells from bone marrow and peripheral blood of rats. Tissue Eng Part A 2012;18:1793-803. [Crossref] [PubMed]
- Longhini ALF, Salazar TE, Vieira C, et al. Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS One 2019;14:e0212642. [Crossref] [PubMed]
- Li S, Huang KJ, Wu JC, et al. Peripheral blood-derived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl Med 2015;4:359-68. [Crossref] [PubMed]
- Fu Q, Tang NN, Zhang Q, et al. Preclinical Study of Cell Therapy for Osteonecrosis of the Femoral Head with Allogenic Peripheral Blood-Derived Mesenchymal Stem Cells. Yonsei Med J 2016;57:1006-15. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 2001;276:37731-4. [Crossref] [PubMed]
- Goncars V, Kalnberzs K, Jakobsons E, et al. Treatment of Knee Osteoarthritis with Bone Marrow–Derived Mononuclear Cell Injection: 12-Month Follow-up. Cartilage 2019;10:26-35. [Crossref] [PubMed]
- Mautner K, Carr D, Whitley J, et al. Allogeneic Versus Autologous Injectable Mesenchymal Stem Cells for Knee Osteoarthritis: Review and Current Status. Tech Orthop 2019;34:244-56. [Crossref]
- Kim SH, Ha CW, Park YB, et al. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 2019;139:971-80. [Crossref] [PubMed]
- Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2016;14:246. [Crossref] [PubMed]
- Jo CH, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med 2017;45:2774-83. [Crossref] [PubMed]
- Emadedin M, Labibzadeh N, Liastani MG, et al. Intra-articular implantation of autologous bone marrow–derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018;20:1238-46. [Crossref] [PubMed]


