
Optimal timing of initiating CRRT in patients with acute kidney injury after liver transplantation
Introduction
Acute kidney injury (AKI) is one of the main postoperative complications after liver transplantation (LT), and is associated with increased morbidity and mortality (1). The incidence of AKI and severe AKI requiring continuous renal replacement therapy (CRRT) were 40.7% and 7.7%, respectively (1). Post-LT AKI is associated with an increased short-term mortality rate and graft dysfunction (2,3). Post-LT AKI is caused by multifactorial origin with recipient, graft, perioperative, and postoperative factors (4). There is increasing evidence that increased model for end-stage liver disease (MELD) score, diabetes mellitus (DM), hypertension, obesity, intraoperative blood loss and transfusion of blood products, postoperative immunosuppression therapy are risk factors (5).
In order to standardize the definition and classification of AKI, the Risk, Injury, Failure, Loss and End-stage (RIFLE) definitions, the Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) criteria were put forward successively (6-8). Although most patients eventually recover after an episode of AKI, many patients invaded their renal reserve or did not return to baseline renal function. And severe AKI requires CRRT, however, there is a lack of consensus on several aspects of CRRT, especially the optimal initiation timing (9). Indeed, despite the large amount of evidence available, it still represents an unsolved problem (10). Especially, there is no report about the optimal initiation timing of CRRT for AKI after LT.
Thus, we aim to estimate the incidence of post-LT AKI in our center and to evaluate its impact on patient outcomes. For severe post-LT AKI patients receiving CRRT treatment, we focus on the effect of the timing of initiation of CRRT on prognosis. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2352).
Methods
Patients who underwent LT between January 2018 to March 2019 at The First Affiliated Hospital, Sun Yat-Sen University (Guangzhou, China) were included. Patients with chronic kidney disease or receiving preoperative CRRT or kidney transplantation before were excluded. Eventually 173 patients were included in this study. Immunosuppressive therapy for all patients after LT was individualized therapy, and immunosuppressive agents were adjusted based on drug blood concentrations.
Recipient data extracted from the medical records included age and gender, model of end-stage liver disease (MELD) score, HBV infection status, previous history of hypertension, DM, previous liver disease, hepatic encephalopathy. For donors, age and gender, body mass index (BMI), donor status (DCD or DBD), cold ischemia time, Pathology of donor liver, serum Na+ and total bilirubin. From the intraoperative period, we recorded: blood loss, volume of blood transfusion, duration of surgery and anhepatic period. Postoperative indicators included: Urine volume, serum creatinine, primary nonfunction (PNF), early allograft dysfunction (EAD), days of intensive care unit (ICU) and overall in-hospital stay, 30 and 90 day mortality rates, as well as follow-up time.
AKI was defined according to KDIGO criteria by serum creatinine and urine output (8). They were categorized as patients with AKI or not. We defined the initiation timing of CRRT based on urine output (during the 24 h prior to CRRT initiation) (11), urine output (UO) criteria were defined as “early” when UO during the 24h prior to CRRT was >0.05 mL/kg/h and as “late” when UO was <0.05 mL/kg/h.
Fisher’s exact test or chi-square test was used for comparisons of categorical data. Student’s t-test and the Mann-Whitney U test were used for the comparisons of continuous variables, according to their distribution, as appropriate. Effects of parameters to estimate AKI were evaluated with multivariate logistic regression models.
All statistical analyses were performed using SPSS version 19.0 statistical software (SPSS, Chicago, IL, USA). Values of P<0.05 were considered to indicate statistical significance.
All organs come from voluntary donation from citizens, no executed prisoner (even with his/her consent) was involved. The study was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (Approval No. (2020) 343) and in accordance with the Declaration of Istanbul. All protocols conformed to the ethical guidelines of the Helsinki Declaration (as revised in 2013).
Results
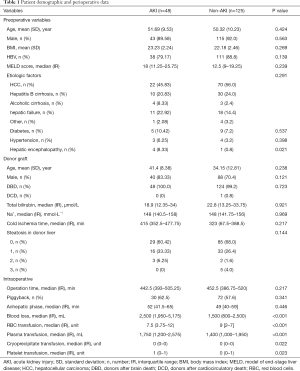
Of the 173 eligible patients, 158 patients (91.33%) were men. The mean age ± SD was 50.88±9.43 years. Hepatocellular carcinoma (HCC) was the most common etiologic factor (53.18%). One hundred and forty-nine patients (86.13%) were hepatitis B positive. the median model of end-stage liver disease (MELD) score prior to LT was 14 (IQR, 9–20.75). There were 14 diabetic patients (8.09%), and 7 patients (4.05%) presented hypertension before LT (Table 1).
Full table
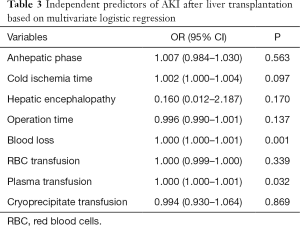
Among the 173 patients, 48 (27.75%) developed AKI after LT. Table 1 shows the risk factors for AKI. Of the 48 AKI patients, 23 received RRT treatment. According to the definition of the initial time of “early” and “late” RRT, 13 patients were divided into the early group and 10 patients into the late group. The clinical differences were compared between early and late group (Table 2). The following factors were AKI predictors: preoperative encephalopathy, high MELD score, intraoperative bleeding volume, Blood transfusion volume, long operation time and cold ischemia time. In the multivariate analysis, independent risk factors for AKI were: intraoperative bleeding volume (OR =1, 95% CI: 1.000–1.001) and plasma transfusion (OR =1, 95% CI: 1.000–1.001) (Table 3).
Full table
Full table
AKI patients had a longer ICU stay, 85 hours (range, 21.5–196.5) vs. 28.6 hours (range, 15–42), P<0.001, as well as longer overall hospital stay, 24.5 days (range, 17–40) vs. 20 days (range, 15–29) (P=0.003), than non-AKI patients, respectively. There were 10 patients (20.83%) and 13 patients (27.08%) died within 30 days and 90 days in the AKI group, while 2 patients (1.6%) and 2 patients (1.6%) died in the non-AKI group. EAD occurred in 25 patients (52.08%) in AKI group and 27 patients (21.6%) in non-AKI group. Primary nonfunction (PNF) occurred in 8 patients (16.67%) in AKI group and 0 patients (0%) in non-AKI group (Table 4).

Full table
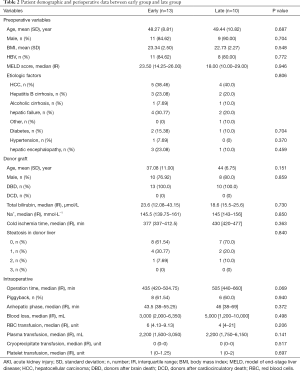
The 30-day mortality was 23.08% in the early group, 40% in the late group. The 90-day mortality was 30.77% in the early group, 50% in the late group. The patients in late group had a longer ICU stay, 183.5 hours (range, 92.25–336.75) vs. 139 hours (range, 94–240), P=0.043, as well as longer overall hospital stay, 38.5 days (range, 17.5–62.75) vs. 35 days (range, 17–38), (P=0.019) than early group patients, respectively. EAD occurred in 9 patients (69.23%) in early group and 8 patients (80%) in late group. Primary non-function (PNF) occurred in 1 patient (7.69%) in early group and 4 patients (40%) in late group. There were 8 patients with severe infection in late group and 4 patients in early group (P=0.026) (Table 5).

Full table
Discussion
LT is the most effective treatment for end-stage liver disease. AKI is a common complication following LT, the incidence ranging from 26.3% to 75.6% in recipients, this disparity is largely due to non-uniform definitions of AKI (12-14). In 2012, KDIGO revised AKI classification merged the AKIN and RIFLE criteria by including both an increase of serum creatinine by ≥26 µmol/L within 48 h as well as an increase to ≥1.5 times baseline within 7 days as threshold for diagnosis of AKI (8). So far, few studies have used KDIGO criteria to evaluate post-LT AKI. The present study used the KDIGO criterion to define and classify AKI, and the incidence of post-LT AKI was 27.75%.
Several risk factors for post-LT AKI have been identified in varying populations. It is likely that post-LT is of multifactorial origin with recipient, graft, perioperative and postoperative factors contributing to its development. Recipient factors included high MELD-scores, pretransplant SCr and BMI (15,16). Intraoperative factors, such as inferior portal vein clamping, intraoperative blood loss and blood transfusion, cold and warm ischemia time, and operation time contributes to AKI occurrence (17). Furthermore, perioperative hyperglycaemia has been suggested as a risk factor for AKI (18). Risk factors for AKI in the post-transplant period included nephrotoxic drugs use, mainly calcineurin inhibitors, and hypoalbuminemia (17,19). In our study, Logistic multivariate analysis suggested that intraoperative bleeding volume and plasma transfusion were risk factors for AKI.
Due to the absence of effective pharmacological treatment, the treatment of AKI patients mainly depends on the management of hemodynamics and volume status, the correction of electrolyte and acid-base disturbances, the provision of adequate nutrition and the adjustment of drug doses. For less severe AKI patients, conservative treatment can be considered as the treatment option. For patients with sustained, severe renal failure, CRRT can be used to treat volume overload, hyperkalemia, acidosis and symptoms of uraemia waiting for the recovery of renal function (20). 20% of AKI patients require CRRT approximately (21). Although survival rates have improved over the past two decades even though the dialysis rate requiring AKI has increased (22), many problems still remain in the optimal administration of CRRT for AKI. Apart from the therapy mode, treatment dose and type of anticoagulation, the initiation timing of treatment is considered an important determinant of the outcome of critically ill patients receiving CRRT (23). The KDIGO AKI guideline is widely accepted and recommend the initiation CRRT without delay in case of life-threatening complications (24). Until recently, only few small RCTs and some observational and cohort studies examining timing of initiation of CRRT, some of which showed beneficial effects of “early” CRRT (25). However, there has been no clear consensus on how to define “timing” relative to the initiation of CRRT in AKI, different definitions of “early” and “late” initial timing of CRRT might have biased the results. Basis of definitions in published studies include urine output, creatinine, urea, time from AKI development and hospital or ICU admission (26,27). In addition, the terms “early” and “late” are relative and what may represent early CRRT in one case could be late in another (28). In our study, we defined “early” and “late” based on urine output, which were defined as “early” when UO during the 24 h prior to CRRT was >0.05 mL/kg/h and as “late” when UO was <0.05 mL/kg/h (11). Timing based on urine output seems to be a more physiological than hospital or ICU admission. Recently, several systematic review and meta-analyses exploring initiation timing of and outcome have been published. The study by Zou et al. (29) strongly supported early initiation, based on the outcomes of 28-day mortality, ICU length of stay, and hospital length of stay. However, there was no difference in survival, ICU or hospital length of stay between early and late CRRT in the studies of Bhatt and Wierstra (26,30). There are a few studies on the application of CRRT in post-LT AKI, but no studies on the prognosis of the initiation timing. According to our study, late initiation of CRRT can prolong ICU and hospital length of stay, and also increase the risk of infection.
This paper had several limitations. This was a retrospective, single-center study with a small number of patients and the short period of time. Thus, there is clearly a requirement for a prospective large scale trial to further understand CRRT for LT-associated AKI in the future.
In conclusion, AKI is a frequent complication of LT, which is associated with higher mortality and longer ICU and hospital stay. The more intraoperative bleeding volume and plasma transfusion are risk factors of post-LT AKI. Late initiation of CRRT can prolong ICU and hospital length of stay, and also increase the risk of infection.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China, China (81873591, and 81670591), Guangdong Natural Science Foundation, China (2016A030311028), The Science and Technology Planning Project of Guangdong Province, China (2018A050506030), and Science and Technology Program of Guangzhou, China (201704020073). The Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007 and 2017B030314018), Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2352
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2352
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-2352
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2352). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki (as revised in 2013) as reflected in a priori approval by the institutional ethics committee [Approval No. (2020) 343]. Because of the retrospective nature, the requirement of informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thongprayoon C, Kaewput W, Thamcharoen N, et al. Incidence and Impact of Acute Kidney Injury after Liver Transplantation: A Meta-Analysis. J Clin Med 2019;8:372. [Crossref] [PubMed]
- Hussaini T, Yoshida EM, Partovi N, et al. Early Persistent Progressive Acute Kidney Injury and Graft Failure Post Liver Transplantation. Transplant Direct 2019;5:e429. [Crossref] [PubMed]
- Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 2015;114:919-26. [Crossref] [PubMed]
- de Haan JE, Hoorn EJ, de Geus HRH. Acute kidney injury after liver transplantation: Recent insights and future perspectives. Best Pract Res Clin Gastroenterol 2017;31:161-9. [Crossref] [PubMed]
- Rahman S, Mallett SV, Davidson BR. Early acute kidney injury after liver transplantation: Predisposing factors and clinical implications. World J Hepatol 2017;9:823-32. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. the ADQI workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care 2004;8:R204. [Crossref] [PubMed]
- Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 2009;35:1692e702.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Section 2: AKI definition. Kidney Int Suppl 2012;2:19-36.
- Romagnoli S, Clark WR, Ricci Z, et al. Renal Replacement Therapy for AKI: When? How much? When to Stop? Best Pract Res Clin Anaesthesiol 2017;31:371-85. [Crossref] [PubMed]
- Pickkers P, Ostermann M, Joannidis M, et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med 2017;43:1198-209. [Crossref] [PubMed]
- Pérez-Fernández X, Sabater-Riera J, Sileanu FE, et al. Clinical variables associated with poor outcome from sepsis-associated acute kidney injury and the relationship with timing of initiation of renal replacement therapy. J Crit Care 2017;40:154-60. [Crossref] [PubMed]
- Kim WH, Lee HC, Lim L, et al. Intraoperative Oliguria with Decreased SvO Predicts Acute Kidney Injury after Living Donor Liver Transplantation. J Clin Med 2018;8:29. [Crossref] [PubMed]
- Jochmans I, Meurisse N, Neirynck A, et al. Hepatic ischemia-reperfusion injury associates with acute kidney injury in liver transplantation: Prospective cohort study. Liver Transplantation 2017;23:634-44. [Crossref] [PubMed]
- Baron-Stefaniak J, Schiefer J, Miller EJ, et al. Comparison of macrophage migration inhibitory factor and neutrophil gelatinase-associated lipocalin-2 to predict acute kidney injury after liver transplantation: An observational pilot study. PLoS One 2017;12:e0183162. [Crossref] [PubMed]
- de Ataide EC, Perales SR, Bortoto JB, et al. Immunomodulation, Acute Renal Failure, and Complications of Basiliximab Use After Liver Transplantation: Analysis of 114 Patients and Literature Review. Transplant Proc 2017;49:852-7. [Crossref] [PubMed]
- Leithead JA, Rajoriya N, Gunson BK, et al. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J Hepatol 2014;60:1180-6. [Crossref] [PubMed]
- Pham PTT, Pham PCT, Wilkinson AH. Management of renal dysfunction in the liver transplant recipient. Curr Opin Organ Transplant 2009;14:231-9. [Crossref] [PubMed]
- Lewandowska L, Matuszkiewicz-Rowińska J, Jayakumar C, et al. Netrin-1 and Semaphorin 3A Predict the Development of Acute Kidney Injury in Liver Transplant Patients. PLoS One 2014;9:e107898. [Crossref] [PubMed]
- Carmona M, Álvarez M, Marco J, et al. Global Organ Transplant Activities in 2015. Data from the Global Observatory on Donation and Transplantation (GODT). Transplantation 2017;101:S29. [Crossref]
- Lins RL. RRT treatment for AKI: is more always better? Nephrol Dial Transplant 2012;27:4252-5. [Crossref] [PubMed]
- Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational Aki-epi study. Intensive Care Med 2015;41:1411-23. [Crossref] [PubMed]
- Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis 2015;65:870-7. [Crossref] [PubMed]
- Vinsonneau C, Allain-Launay E, Blayau C, et al. Renal replacement therapy in adult and pediatric intensive care. Ann Intensive Care 2015;5:58. [Crossref] [PubMed]
- Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for Acute Kidney Injury. Am J Kidney Dis 2013;61:649-72. [Crossref] [PubMed]
- Meersch M, Küllmar M, Schmidt C, et al. Long-Term Clinical Outcomes after Early Initiation of RRT in Critically Ill Patients with AKI. J Am Soc Nephrol 2018;29:1011-9. [Crossref] [PubMed]
- Wierstra BT, Sameer K, Soha A, et al. The impact of “ early” versus “ late “ initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care 2016;20:122. [Crossref] [PubMed]
- Liu Y, Davari-Farid S, Arora P, et al. N. D. Early Versus Late Initiation of Renal Replacement Therapy in Critically Ill Patients With Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-analysis. J Cardiothorac Vasc Anesth 2014;28:557-63. [Crossref] [PubMed]
- Romagnoli S, Ricci Z. When to start a renal replacement therapy in acute kidney injury patients (AKI): many irons in the fire. Ann Transl Med 2016;4:355. [Crossref] [PubMed]
- Zou H, Hong Q, Xu G. Early versus late initiation of renal replacement therapy impacts mortality in patients with acute kidney injury post cardiac surgery: a meta-analysis. Crit Care 2017;21:150. [Crossref] [PubMed]
- Bhatt GC, Das RR. Early versus late initiation of renal replacement therapy in patients with acute kidney injury-a systematic review & meta- analysis of randomized controlled trials. BMC Nephrol 2017;18:78. [Crossref] [PubMed]


