
Metformin inhibits inflammation and bone destruction in collagen-induced arthritis in rats
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that is mainly characterized by progressive arthritis leading to joint damage and dysfunction. Several studies have shown that the secretion of proinflammatory cytokines and bone destruction are closely related to the pathogenesis of RA (1). A large number of studies have found that interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) play important roles in the pathogenesis of RA (2). Terkeltaub et al. found that IL-1β and TNF-α can induce cartilage destruction and precipitate bone destruction (3,4). In joints, the balance between bone formation by osteoblasts and bone resorption by osteoclasts is involved in maintaining bone homeostasis (5). The inflammatory microenvironment can activate bone resorption and inhibit the reparative effect of osteoblasts, leading to bone erosion. Danks et al. found that IL-1β and TNF-α can alter bone homeostasis and cause bone destruction (6). Therefore, proinflammatory cytokines and bone homeostasis contribute significantly to the progression of RA.
Metformin (MF) is a common oral hypoglycemic agent mainly used to treat type 2 diabetes. In patients with RA, glucose metabolism affects disease progression (7); it can be reasoned that optimizing glucose metabolism may help to control RA activity. Son et al. found that MF can reduce the inflammation of arthritis by regulating the T helper 17/regulatory T cell (Th17/Treg) balance (8). Furthermore, Chen et al. found that MF can inhibit the proliferation of RA fibroblast-like synoviocytes through the insulin-like growth factor receptor (IGF-IR)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway (9). Several studies in vitro have found that MF can inhibit osteoclast activation and induce osteoblast differentiation (10-12). Conversely, MF has also been observed to inhibit osteoblast differentiation (13). Therefore, the mechanism of MF on RA requires further investigation.
Collagen-induced arthritis (CIA) is the most widely used model to simulate the development of RA. In our study, we investigated the protective effects of MF on systemic inflammation and joints in CIA rats. Detection of serum-related inflammatory factors, histological and immunohistochemical analysis of knee joints, and detection of the expression of related genes in cartilage were performed to elucidate whether MF may be a potential therapeutic agent for RA.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3042).
Methods
Animals
Male Wistar rats (7–8 weeks old) were provided by the Laboratory Animal Center of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University Hospital. They were kept in a specialized environment with independent ventilated cages (IVC) in specific-pathogen-free (SPF) facilities with pathogen-free conditions. The experimental protocol was approved by the Ethics Committee of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine {No. HKDL[2017]207}, in compliance with institutional guidelines for the care and use of animals.
Drugs and reagents
MF was purchased from Bristol-Myers Squibb (Shanghai, China). The 2 mg/mL bovine type II collagen (CII) and incomplete Freund’s adjuvant (IFA) were purchased from Chondrex (Redmond, WA, USA). The primary antibodies of matrix metallopeptidase 9 (MMP-9) and a disintegrin and metalloproteinase with thrombospondin-like motifs 5 (ADAMTS-5) were purchased from Cell Signaling Technology (CST) (Danvers, MA, USA). Rat enzyme-linked immunosorbent assay (ELISA) kits (TNF-α and IL-1β) were purchased from Multi Sciences (Hangzhou, Zhejiang, China). The TRIzol reagent was purchased from Ambion (Shanghai, China). The complementary DNA (cDNA) reverse transcription kit was purchased from Takara Bio (Kusatsu, Shiga, Japan).
Experimental protocols
We randomly allocated 36 rats into equal groups of three groups: a normal group, a CIA group, and CIA treated with MF group. With the exception of the normal group, the other two groups of rats were immunized. On day 0, IFA and CII were emulsified at 1:1 and then injected intradermally into the base of the rats’ tails. On day 7, the same emulsion was injected in the same way. Beginning on the 14th day, MF (100 mg/kg) was injected intraperitoneally into CIA rats every 3rd day for 3 weeks.
Pharmacodynamic evaluation
After the first immunization, two independent observers monitored the clinical symptoms of the rats twice a week and measured the volume of the hind paws. At the same time, the severity of arthritis on each paw of the rats was scored from 0 to 4 according to the following system: 0, normal; 1, mild swelling; 2, moderate swelling at the ankle; 3, obvious swelling, including ankles, feet, and toes; 4, severe arthritis involving the entire paw.
Determination of TNF-α and IL-1β in peripheral blood
At the end of the experiment, all rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (45 mg/kg) and then killed by cervical dislocation. The upper plasma was obtained after peripheral was blood taken and centrifuged at 3,500 rpm for 10 min at 4 °C. The levels of cytokines (IL-1β, TNF-α) in the rat plasma were measured according to the instructions of the ELISA kit.
Microscopic computed tomography (micro-CT) examination
The hind limbs of the rats were dissected and fixed in 4% paraformaldehyde (PFA), and then subjected to micro-CT analysis using the Inveon Micro positron emission tomography (PET)/CT system (Siemens Co., Knoxville, TN, USA). A tomographic scan of the hind limbs was performed with a custom resolution of 10 µm, a voltage of 70 kV, and a current of 114 mA. We reconstructed and analyzed the three-dimensional (3D) structure and morphometry in a double-blind manner. The area between the proximal tibia and the distal femur was selected for analysis of the following parameters: trabecular bone volume fraction (Trab BV/TV), trabecular spacing (Tb.Sp), trabecular number (Tb.N), and trabecular thickness (Tb.Th).
Histopathological analysis of the knee joint
The hind legs were separated and placed in 4% PFA for 24 h, and then decalcified with 0.5 M ethylenediamine tetraacetic acid (EDTA) and embedded in paraffin. Paraffin sections with a thickness of 5 µm were prepared, dewaxed with xylene, gradiently dehydrated with ethanol, and finally subjected to hematoxylin and eosin (HE), tartrate resistant acid phosphatase (TRAP), alcian blue, and toluidine blue staining. Synovial hyperplasia, inflammatory infiltration, and neovascularization in the knee joint were analyzed by HE staining. The number and area of osteoblasts and osteoclasts in the knee joint were observed by HE and TRAP staining to evaluate the bone destruction of the joint. The cartilage degradation in the knee joint was analyzed by alcian blue and toluidine blue staining.
Immunohistochemistry
The slices were dewaxed in xylene and hydrated with gradient alcohol. Proteinase K digestion buffer was added to repair the antigen, and 3% hydrogen peroxide was added to block endogenous peroxidase. Next, they were blocked with 5% bovine serum albumin (BSA) and then incubated overnight with the primary antibodies (MMP-9, ADAMTS-5) at 4 °C. The slices were then washed of the primary antibodies and incubated with the secondary antibody at room temperature for 30 min; Dolichos bifloris agglutinin (DBA) was then added for color development. The slides were restained with Mayer’s hematoxylin and then installed. The expression level of antibodies in articular cartilage was evaluated by two independent observers.
Real-time polymerase-chain reaction (RT-PCR)
Total RNA was extracted from the rat femoral head using the TRIzol reagent, and the concentration and purity were measured. The cDNA reverse transcription kit was used to reverse-transcribe into cDNA according to standard procedures. The RT-PCR primers were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. The primers were as follows: caspase-3 (Casp3) (F): 5'-GGAGCTTGGAACGCGAAGAA-3' and (R): 5'-ACACAAGCCCATTTCAGGGT-3'; Bcl-2 associated X protein (Bax) (F): 5'-TTGCTACAGGGTTTCATCCAGG-3' and (R): 5'-CACTCGCTCAGCTTCTTGGT-3'; protein 53 (p53) (F): 5'-CCCCTGAAGACTGGATAACTGT-3' and (R): 5'-CACTTGGAGGGCTTCCTCTG-3'. Gene expression levels of Casp3, Bax, and p53 were measured via RT-PCR (ABI 7500, Applied Biosystems Inc., USA), according to the manufacturer’s protocols.
Statistical analysis
The results are expressed as mean ± standard deviation (SD). The statistical significance between various groups was analyzed using one-way analysis of variance (ANOVA) in SPSS 22.0 software. Differences between groups of P<0.05 were considered statistically significant.
Results
MF inhibited the progression of arthritis and systemic inflammation in CIA rats
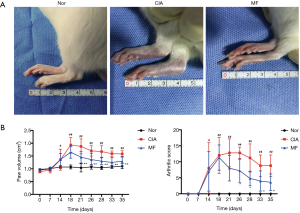
No rat death was observed during the course of the experiment. Beginning the 10th day, the paws of the immunized rats presented as obviously red and swollen and reached a peak on the 18th day. Beginning the 14th day, MF was injected intraperitoneally into the CIA rats every 3 days for 3 weeks. Following treatment with MF, arthritis symptoms were significantly reduced (Figure 1A). The CIA group showed significant paw swelling from day 14. After administration of MF, the ankle joint swelling was significantly reduced from day 21 (Figure 1B). As revealed by the arthritic score, the arthritis symptoms of the CIA group were apparent on the 14th day and reached a peak at around day 21. After MF treatment, arthritic symptoms were significantly relieved from day 26 (Figure 1B). After completion of administration, the levels of pro-inflammatory cytokines (IL-1β, TNF-α) in the peripheral plasma were measured by ELISA test. The levels of IL-1β and TNF-α in the peripheral blood of the CIA group were significantly increased, but they were significantly reduced in the MF group (Figure 2A). Therefore, these results demonstrate that MF has a significant inhibitory effect on arthritis symptoms and systemic inflammation in CIA rats.
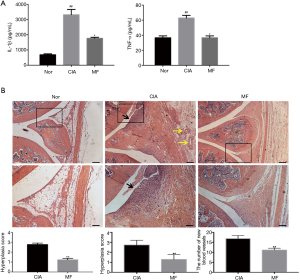
MF inhibited synovial hyperplasia, inflammatory infiltration, and neovascularization in CIA rats
Compared with the joints of the normal rats, CIA rats showed obvious symptoms of RA, such as synovial hyperplasia, inflammatory cell infiltration, and neovascularization. Histological analysis found that MF improved arthritis symptoms in CIA rats. The histological score of the synovium showed that MF significantly inhibited synovial hyperplasia, inflammatory infiltration, and neovascularization in CIA rats (Figure 2B).
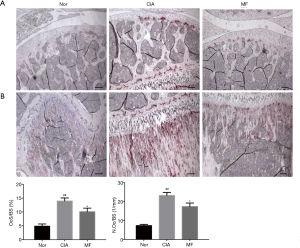
MF improved the joint bone destruction in CIA rats
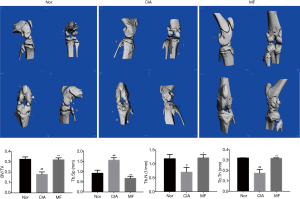
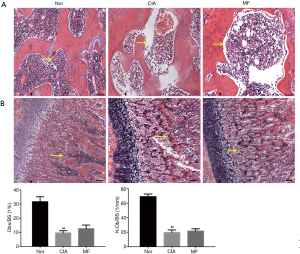
Compared with the joints of normal rats, there was significant bone destruction in the knee joints of CIA rats. After treatment with MF, the bone destruction phenomenon was significantly improved. From the parameter analysis of rat knee micro-CT, it could be seen that the values of BV/TV, Tb.N and Tb.Th of the CIA group were downregulated, and the values of Tb.Sp were upregulated. After 3 weeks of MF treatment, the values of BV/TV, Tb.N, and Tb.Th were significantly upregulated, and the values of Tb.Sp were significantly reduced (Figure 3). Osteoblasts play a very important role in regulating bone metabolism. Compared to normal rats, there was a significant decline in osteoblasts in the region of knee joint in CIA rats. There was no significant improvement observed in osteoblast loss in the regions of subchondral bone and trabecular bone after the administration of MF (Figure 4). Osteoclast formation is a major problem in RA. Compared to normal rats, there was a significant increase in osteoclasts in the knee joint region of CIA rats. After treatment with MF, the proliferation of osteoclasts in cartilage and trabecular bone was significantly alleviated (Figure 5). Therefore, MF can play a bone protective role by inhibiting osteoclast formation.
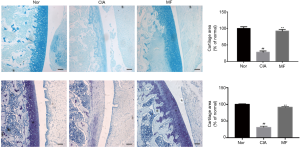
MF inhibited cartilage destruction in CIA rats
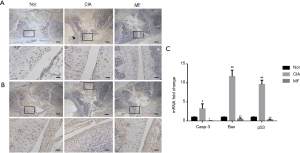
Damage of the articular cartilage is another important factor that contributes to bone destruction. Compared to normal rats, there was significant degradation of the cartilage layer of the knee joint in CIA rats. After treatment with MF, the cartilage damage was significantly alleviated (Figure 6). The roles of enzymes MMP-9 and ADAMTS-5 are very important in the degradation of articular cartilage. Compared with the normal group, the expression of MMP-9 and ADAMTS-5 in cartilage of CIA rats was significantly increased, but was decreased following treatment with MF (Figure 7A,B). Casp3, Bax and p53, are important in regulating apoptosis of chondrocytes. Accordingly, we analyzed their expression in chondrocytes by RT-PCR. Compared to normal rats, the mRNA expression of Casp3, Bax, and p53 in the chondrocytes of CIA rats was significantly higher (Figure 7C). Nevertheless, the mRNA expression of Casp3, Bax, and p53 was significantly decreased after MF treatment. Therefore, MF was seen to have a protective effect against apoptosis of chondrocytes in CIA rats.
Discussion
In the current study, we used the established CIA model to study the effects of MF on inflammation and bone destruction in arthritis. Inflammation and joint destruction caused by synovitis are the main features of RA (14). The synovial tissue of RA joints showed significant synovial hyperplasia, inflammatory infiltration, and neovascularization (15). In addition, the breakdown of the synovial membrane can lead to cartilage destruction and bone erosion (16). Multiple studies have found that MF can reduce arthritis inflammation by regulating the Th17/Treg balance (8). However, studies on the protective effects of MF on bone and cartilage conferred by RA are still relatively few. In our collaborative research with Li et al., we found that MF can significantly limit the development and progress of osteoarthritis to aid in bone protection (17). Therefore, it is interesting to study the role of MF in protecting bone in RA. In our study, we found that MF-treated CIA rats had significantly reduced paw volume and arthritis scores. In previous studies, we found a significant increase in inflammatory cytokines in the peripheral blood of CIA rats (18). In this study, systemic inflammation mediated by cytokines (including IL-1β and TNF-α) in the peripheral blood of CIA rats after MF treatment was controlled. Histological analysis of the knee joint revealed that synovial hyperplasia was suppressed, inflammatory infiltration was reduced, and neovascularization was controlled after MF treatment. In summary, our results indicated that MF can significantly inhibit systemic inflammation and synovitis in CIA rats.
In addition to inflammation, CIA rats also exhibit severe bone erosion. In order to determine whether MF can protect bones against the erosion caused by arthritis, we analyzed the knee joint microstructure by micro-CT and histomorphometry. Studies have reported that the number, volume, and thickness of trabeculae of CIA rats are significantly reduced, and the distance between trabeculae is increased. In our study, after MF treatment was applied, the CIA-related bone destruction was significantly suppressed. The severity of bone destruction is closely related to the balance between osteoblasts and osteoclasts (19). Staining of the knee joint with HE revealed that severe osteoblast loss had occurred in the subchondral bone and trabecular bone area of CIA rats, and it was not significantly improved after MF treatment. Staining of the knee joint with TRAP revealed that osteoclasts in the subchondral bone and trabecular bone region of CIA rats were significantly increased, and were significantly inhibited after MF treatment. Therefore, our results indicated that MF can exert bone protection by inhibiting osteoclast formation.
Damage to the articular cartilage is also an important cause of bone destruction (20,21). Cartilage is mainly composed of chondrocytes and extracellular matrix (ECM). The composition of ECM is mainly interstitial fluid, collagen, and proteoglycans. Under physiological conditions, the balance of chondrocytes and ECM maintains the homeostasis of cartilage (22). However, in the case of RA, this steady state is interrupted. Alcian blue and toluidine blue staining of the knee joints revealed that the cartilage layer of CIA rats had become significantly degraded, and was improved after treatment with MF. In cartilage damage, ADAMTS and matrix metalloproteinases (MMPs) all play important roles in the degradation of ECM (23). The most important enzyme in MMPs is MMP-9; it is highly expressed in arthritis, can specifically degrade collagen, and its reduction can ameliorate the progress of arthritis (24,25). ADAMTS-5 is considered the most important enzyme for degrading proteoglycans in ADAMTS (26). Chen et al. found that ADAMTS-5 inhibitors can significantly inhibit cartilage degeneration in arthritis (27). In our study, the expression of MMP-9 and ADAMTS-5 in the cartilage layer of CIA rats was increased, and was then significantly inhibited after MF treatment. In addition to the degradation of ECM, chondrocyte apoptosis causes cartilage degeneration (28). Bax is a well-known pro-apoptotic protein of the B-cell lymphoma-2 (Bcl-2) family, and is thought to play an important role in chondrocyte apoptosis (28). Belonging to the cysteine protease family, Casp3 is considered to be an important mediator of nitric oxide (NO)-induced chondrocyte apoptosis (29). The tumor suppressor gene p53 plays a vital role in regulating bone and chondrocyte apoptosis (30). In our study, the expression of related pro-apoptotic genes (Bax, Casp3, and p53) in chondrocytes in CIA rats was significantly increased, and the viability of chondrocytes was significantly increased after MF treatment. In summary, our results indicate that MF can significantly inhibit cartilage degeneration induced by CIA.
Conclusions
Overall, this study clearly showed that MF can inhibit systemic inflammation and synovitis, and protect bone by inhibiting ECM degradation, osteoclast formation, and chondrocyte apoptosis. Our results suggest that MF may be a potential drug for the treatment of RA.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China [Grants 81874011, 81572104, and 81301531 (to TYW)]. This work was also partially supported by the Shanghai Municipal Science and Technology Commission [Innovation Grant 18140903502 (to TYW)].
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3042
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3042
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3042). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experimental protocol was approved by the Ethics Committee of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine {No. HKDL[2017]207}, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hammaker D, Firestein GS. Epigenetics of inflammatory arthritis. Curr Opin Rheumatol 2018;30:188-96. [Crossref] [PubMed]
- Huang CC, Chiou CH, Liu SC, et al. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J Pineal Res 2019;66:e12560. [Crossref] [PubMed]
- Terkeltaub R, Yang B, Lotz M, et al. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum 2011;63:1928-37. [Crossref] [PubMed]
- Petursson F, Husa M, June R, et al. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res Ther 2013;15:R77. [Crossref] [PubMed]
- Tanaka Y, Okada Y, Nakamura T. Inter- and intracellular signaling in secondary osteoporosis. J Bone Miner Metab 2003;21:61-6. [Crossref] [PubMed]
- Danks L, Komatsu N, Guerrini MM, et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis 2016;75:1187-95. [Crossref] [PubMed]
- Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum 2006;54:2765-75. [Crossref] [PubMed]
- Son HJ, Lee J, Lee SY, et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm 2014;2014:973986. [Crossref] [PubMed]
- Chen K, Lin ZW, He SM, et al. Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed Pharmacother 2019;115:108875. [Crossref] [PubMed]
- Shah M, Kola B, Bataveljic A, et al. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone 2010;47:309-19. [Crossref] [PubMed]
- Lee YS, Kim YS, Lee SY, et al. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone 2010;47:926-37. [Crossref] [PubMed]
- Wang P, Ma T, Guo D, et al. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J Tissue Eng Regen Med 2018;12:437-46. [Crossref] [PubMed]
- Zhen D, Chen Y, Tang X, et al. Metformin reverses the deleterious effects of high glucose on osteoblast function. J Diabetes Complications 2010;24:334-44. [Crossref] [PubMed]
- Karouzakis E, Gay RE, Gay S, et al. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nat Rev Rheumatol 2009;5:266-72. [Crossref] [PubMed]
- Leblond A, Allanore Y, Avouac J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun Rev 2017;16:594-601. [Crossref] [PubMed]
- García-Vicuña R, Gómez-Gaviro MV, Domínguez-Luis MJ, et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum 2004;50:3866-77. [Crossref] [PubMed]
- Li J, Zhang B, Liu WX, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signaling. Ann Rheum Dis 2020;79:635-45. [Crossref] [PubMed]
- Wu J, Zhao FT, Fan KJ, et al. Dihydromyricetin inhibits inflammation of fibroblast-like synoviocytes through regulation of nuclear factor-κb signaling in rats with collagen-induced arthritis. J Pharmacol Exp Ther 2019;368:218-28. [Crossref] [PubMed]
- Rajaei E, Haybar H, Mowla K, et al. Metformin one in a million efficient medicines for rheumatoid arthritis complications: inflammation, osteoblastogenesis, cardiovascular disease, malignancies. Curr Rheumatol Rev 2019;15:116-22. [Crossref] [PubMed]
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023-38. [Crossref] [PubMed]
- Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis--two unequal siblings. Nat Rev Rheumatol 2015;11:606-15. [Crossref] [PubMed]
- Yasuda T. Cartilage destruction by matrix degradation products. Mod Rheumatol 2006;16:197-205. [Crossref] [PubMed]
- Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev 2008;223:252-70. [Crossref] [PubMed]
- Tchetverikov I, Ronday HK, Van EIB, et al. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis 2004;63:881-3. [Crossref] [PubMed]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: Role in arthritis. Front Biosci 2006;11:529-43. [Crossref] [PubMed]
- Stanton H, Rogerson FM, East CJ, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 2005;434:648-52. [Crossref] [PubMed]
- Chen P, Zhu S, Wang Y, et al. The amelioration of cartilage degeneration by ADAMTS-5 inhibitor delivered in a hyaluronic acid hydrogel. Biomaterials 2014;35:2827-36. [Crossref] [PubMed]
- Miao G, Zang X, Hou H, et al. Bax targeted by miR-29a regulates chondrocyte apoptosis in osteoarthritis. Biomed Res Int 2019;2019:1434538. [Crossref] [PubMed]
- Ueng SW, Yuan LJ, Lin SS, et al. Hyperbaric oxygen treatment prevents nitric oxide-induced apoptosis in articular cartilage injury via enhancement of the expression of heat shock protein 70. J Orthop Res 2013;31:376-84. [Crossref] [PubMed]
- Liu B, Lei M, Hu T, et al. Inhibitory effects of SRT1720 on the apoptosis of rabbit chondrocytes by activating SIRT1 via p53/bax and NF-κB/PGC-1α pathways. J Huazhong Univ Sci Technolog Med Sci 2016;36:350-5. [Crossref] [PubMed]
(English Language Editors: J. Jones and J. Gray)


