Regulatory T cell and activated natural killer cell infiltration in hepatocellular carcinoma: immune cell profiling using the CIBERSORT
Introduction
Hepatocellular carcinoma (HCC) is understood to be an immunogenic tumor caused by chronic liver disease. HCC develops when the liver becomes chronically inflamed and the intra-hepatic immunosuppressive microenvironment occurs (1-3). Emerging research has indicated close interaction between various immune cells and tumor cells. Many molecular mechanisms involved in the biological properties of tumor cells during hepatocarcinogenesis also have considerable implications on the immune system (4).
Although a variety of tumor-associated antigens are expressed in HCC, no immune therapies have proved beneficial in the treatment of HCC (5). Several novel immunotherapeutic approaches have been trialed and have shown promising efficacy (6,7). However, the efficacy of immunotherapies in HCC is limited by several factors. More focused studies into how tumor response can be accurately assessed and predicted, as well as how the immunosuppressive effects of the tumor microenvironment can be overcome, are urgently needed (8,9). Immunophenotyping, which has shown potential predictive value for the prognosis of various human malignancies (10,11), might allow responsive and non-responsive patients to be identified based on the extent and distribution of immune cell infiltration (10,12,13). Bioinformatic analysis of tumor-infiltrating immune cells in HCC tissues have been addressed in previous studies (14,15). In a study reported by Rohr-Udilova et al., total B cells, memory B cells, T follicular helper cells and M1 macrophages were strongly infiltrated into HCC (14). Since the efficacy of immunotherapy for HCC is not satisfactory (16), the supplementary assessment of tumor-infiltrating immune cells should be addressed further.
Recent reports have demonstrated that deconvolution of gene expression data by Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts (CIBERSORT) can provide crucial information regarding immune cell composition in HCC (14,17). In this analysis, we selected microarray dataset GSE84402 with 14 paired tumor and the corresponding non-cancerous tissues and aimed to investigate the relative proportions and distribution of tumor-infiltrating immune cells in HCC patients using CIBERSORT. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5830).
Methods
Data source
The GSE84402 series comprising 14 pairs of HCC tissues and the corresponding non-cancerous tissues was obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). Double-stranded complementary DNA (cDNA) was synthesized using 5 µg of total RNA from each sample, before amplification, labeling with biotin, and hybridization to GeneChip Human Genome U133 Plus 2.0 Array (18). As declared by Wang et al., ethical approval was obtained from the Zhongshan Hospital Research Ethics Committee, and written informed consent was obtained from each patient (18).
The GSE84402 series matrix file and platform documents (GPL570) were downloaded. The Perl programming language was used to translate gene probe IDs to gene symbol ID. The limma package (19,20) in R program was used to normalized the gene expression levels of the tumor and non-tumor tissue samples from HCC patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
CIBERSORT analysis
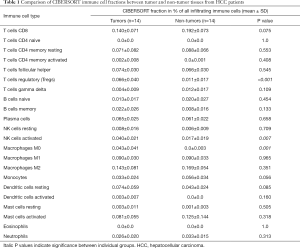
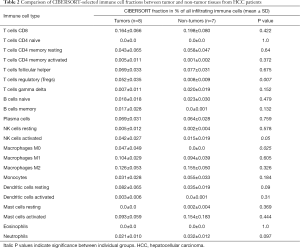
Cell-type Identification by Estimating Relative Subsets of RNA Transcripts (CIBERSORT), created by Newman et al., is an analytical tool that allows the abundance of member cell types in a mixed cell population to be estimated using gene expression data (17). CIBERSORT gene signature matrix, termed LM22, contains 547 genes and distinguishes 22 human hematopoietic cell phenotypes, including 7 T cell types, naïve and memory B cells, plasma cells, NK cells, and myeloid subsets. CIBERSORT was used to obtain the immune cell profile of each sample from GSE84402, with the number of permutations set at 100 (17). Table 1 shows the complete samples from GSE84402. For each sample, 22 types of immune cell, along with CIBERSORT metrics including the Pearson correlation coefficient, CIBERSORT P value, and root mean squared error (RMSE), were quantified. The CIBERSORT P value represents the statistical significance of the deconvolution results across all cell subsets and can be used to filter out deconvolution with less significant fitting accuracy. From the samples analyzed, 8 non-tumor samples and 7 tumor samples were identified when a CIBERSORT P value ≤0.05 was required. Table 2 lists the samples selected. SPSS 23.0 software (SPSS Inc., Chicago, USA) was employed to compare CIBERSORT immune cell fractions between tumor and non-tumor tissues from HCC patients.
Full table
Full table
The pheatmap package in R program, (https://cran.r-project.org/web/packages/pheatmap/) was used to perform heatmap analysis of immune cells in tumor and non-tumor tissues. Correlation analysis of immune cells was conducted using the corrplot package (https://cran.r-project.org/web/packages/corrplot/index.html). Four paired samples from the samples selected by CIBERSORT were used to calculate the difference between candidate infiltrating immune cells.
Results
Comparison of CIBERSORT immune cell fractions
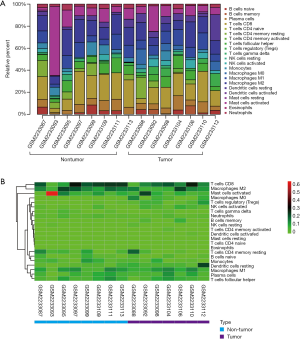
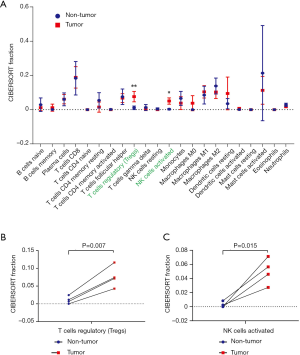
The CIBERSORT immune cell fractions of the tumor and non-tumor tissues from HCC patients from GSE84402 were compared (Table 1). The tumor tissues showed significant infiltration by regulatory T cells (Tregs), activated natural killer (NK) cells, and M0 macrophages compared to non-tumor tissues (P<0.001, P=0.007, and P=0.001, respectively, Table 1 and Figure 1A).
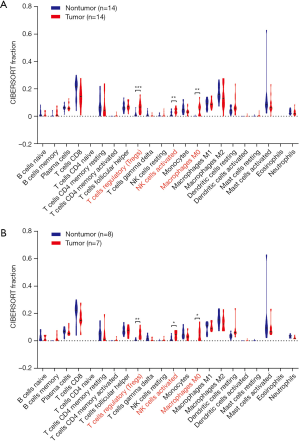
From the samples analyzed, 8 non-tumor and 7 tumor samples were selected when the CIBERSORT P value was set at ≤0.05. Table 2 compares the CIBERSORT immune cell fractions of the tumor and non-tumor tissues from HCC patients. Consistent with above, there was significant infiltration by regulatory T cells, activated NK cells, and M0 macrophages in HCC tumor tissues (P=0.007, P=0.05, and P=0.025, respectively; Table 2 and Figure 1B). The landscape and heatmap of immune cells in the tumor and non-tumor tissue samples are presented in Figure 2.
Comparison of CIBERSORT immune cell fractions in paired samples
Four paired tumor and non-tumor samples from HCC patients (GSM2233092/GSM2233093, GSM2233098/GSM2233099, GSM2233110/GSM2233111, and GSM2233112/GSM2233113) were identified from the samples selected by CIBERSORT. As shown in Figure 3, infiltration by regulatory T cells and activated NK cells was more significant in tumor tissues than in non-tumor tissues (P<0.01 and P<0.05, respectively; Figure 3A). Moreover, the levels of regulatory T cells and activated NK cells were significantly increased in each tumor tissue sample compared with those in the corresponding non-tumor tissue sample (P=0.007 and P=0.015, respectively; Figure 3B,C).
Correlation of immune cells
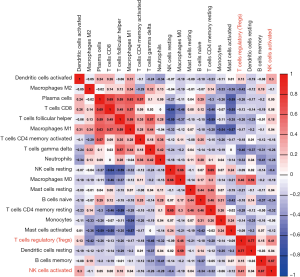
Figure 4 shows a correlation map displaying the Pearson correlation values for each comparison between the immune cells in the CIBERSORT-selected samples. As shown in Figure 4, regulatory T cells had a positive Pearson correlation value of 0.77 with resting dendritic cells and 0.46 with macrophages M0 cells. Furthermore, activated NK cells had positive Pearson correlation values of 0.67 and 0.41 with memory B cells and regulatory T cells, respectively.
Discussion
Several computational tools, including microarray microdissection with analysis of differences (MMAD) (1,21), linear least-square regression (LLSR) (22) and digital sorting algorithm (DSA) (23), have been used to deconvolute complex gene expression profiles mixtures to infer cellular composition. However, their sensitivities to experimental noise and cell types, and high unknown mixture content limited the utility for tumor infiltrating immune cells assessment. CIBERSORT is a widely useful approach for high throughput characterization of tumor infiltrating immune cells assessment from complex tissues (24).
Tumor-infiltrating immune cells have been associated with the aggressiveness of many cancers (25,26), and their prognostic correlation with HCC has been extensively investigated (14,27,28). In HCC patients, abundant T lymphocytes (29,30), B cells (31), NK cells (32), and dendritic cells (33,34) have been found to contribute to favorable prognosis. In contrast, high levels of regulatory T cells (35), neutrophils (36,37), monocytes (38), and CXCR3+ subtype B cells (39) have been associated with poor outcomes in HCC patients. Moreover, a link has been reported between the proportions of different types of macrophages and the prognosis of HCC (40,41). Therefore, uncovering the diverse composition of tumor-infiltrating immune cells in tumor immunology might be helpful in overcoming the limitations and barriers faced by immunotherapies in HCC (42).
In this analysis, we found significant infiltration by Tregs and activated NK cells in HCC tumor tissues. In the GSE84402 series, 13 out of 14 cases were HBsAg positive. Consistent with our findings, a previous study revealed that Tregs in tumor tissues displayed higher frequencies and more suppressive phenotypic functions than those in peritumoral and peripheral tissues (43). The higher Treg levels in patients with chronic hepatitis B (CHB) exerted a suppressive effect on the specific immune responses induced by HBV antigens, as well as by HCC tumor antigens. Furthermore, Tregs could inhibit tumor immuno-surveillance against HCC, which potentially plays a role in the immunopathogenesis from CHB to HCC (44). Tregs have crucial involvement in forming and maintaining the hepatic inhibitory microenvironment, as well as in the development of cirrhosis, the transformation of cirrhosis to HCC, and HCC progression and metastasis (45). Moreover, various studies have demonstrated that high quantities of tumor-infiltrating Tregs are considered to be an unfavorable prognostic indicator for advanced tumor biological behaviors and poor survival in HCC patients (46-49).
NK cell regulation of antigen-specific T lymphocytes occurs during viral infection. When NK cells become depleted, the number of antigen-specific T lymphocytes increases; therefore, these NK cells may reduce T cell levels, leading to a weaker antitumor immune response (50). NK cell activator has been shown to induce liver inflammation by significantly increasing the levels of infiltrating lymphocytes, along with concurrently increasing apoptosis and proliferation of hepatocytes; consequently, epithelial-to-mesenchymal transition of hepatocytes is accelerated (51). Decreased NK cell counts have also been reported in HCC patients (52). NK cell deficiency is believed to be a key mechanism underlying tumor cell evasion of the host’s immune system. In HCC, NK cells appear to have reduced function. A growing bank of evidence suggests that impaired NK cell function leads to the body’s failure to eliminate tumor cells, which indicates that tumor cells could be killed more effectively through enhancing the activity of dysfunctional NK cells (32,53). Our results also showed that Tregs were positively correlated with activated NK cells. Current evidence showed that Tregs are closely linked to the homeostasis of NK cells and attenuate the sensitivity of target cells through minimizing interleukin (IL)-2 to NK cells (50). In contrast, in chronic myeloid leukemia, the degree of NK cell differentiation was closely and inversely correlated with the proportion of Treg cells (54). Considered these conflicting results regarding the correlation of tumor-infiltrating NK cells with Tregs, more mechanism investigations on tumor-infiltrating NK cells in HCC development should be carried out in future.
Our study has some limitations. The primary is that this study is based on relatively small samples, the external validation of our results should be considered. Secondly, the associations between Tregs and NK cells and prognosis in HCC patients were not addressed in this study. Thirdly, this study was a preliminary bioinformatic analysis, no experimental data was available. Even though, the CIBERSORT approach to analyzing HCC samples from the GSE84402 series revealed that tumor tissues were markedly infiltrated by Tregs and activated NK cells. Therefore, these cells should be considered as candidate therapeutic targets in multidisciplinary treatment for HCC.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (81803901).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5830
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5830). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mahipal A, Tella SH, Kommalapati A, et al. Immunotherapy in hepatocellular carcinoma: is there a light at the end of the tunnel? Cancers (Basel) 2019;11:1078. [Crossref] [PubMed]
- Buonaguro L, Mauriello A, Cavalluzzo B, et al. Immunotherapy in hepatocellular carcinoma. Ann Hepatol 2019;18:291-7. [Crossref] [PubMed]
- Tan KW, Chacko AM, Chew V. PD-1 expression and its significance in tumour microenvironment of hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2019;4:51. [Crossref] [PubMed]
- Tang X, Shu Z, Zhang W, et al. Clinical significance of the immune cell landscape in hepatocellular carcinoma patients with different degrees of fibrosis. Ann Transl Med 2019;7:528. [Crossref] [PubMed]
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015;12:681-700. [Crossref] [PubMed]
- Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open 2018;3:e000455. [Crossref] [PubMed]
- Siu EH, Chan AW, Chong CC, et al. Treatment of advanced hepatocellular carcinoma: immunotherapy from checkpoint blockade to potential of cellular treatment. Transl Gastroenterol Hepatol 2018;3:89. [Crossref] [PubMed]
- Xu W, Liu K, Chen M, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol 2019;11:1758835919862692. [Crossref] [PubMed]
- Tian M, Shi Y, Liu W, et al. Immunotherapy of hepatocellular carcinoma: strategies for combinatorial intervention. Sci China Life Sci 2019;62:1138-43. [Crossref] [PubMed]
- Gnjatic S, Bronte V, Brunet LR, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer 2017;5:44. [Crossref] [PubMed]
- Mlecnik B, Bindea G, Angell HK, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016;44:698-711. [Crossref] [PubMed]
- Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic Changes in PD-L1 Expression and Immune Infiltrates Early During Treatment Predict Response to PD-1 Blockade in Melanoma. Clin Cancer Res 2017;23:5024-33. [Crossref] [PubMed]
- Ribas A, Shin DS, Zaretsky J, et al. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol Res 2016;4:194-203. [Crossref] [PubMed]
- Rohr-Udilova N, Klinglmuller F, Schulte-Hermann R, et al. Deviations of the immune cell landscape between healthy liver and hepatocellular carcinoma. Sci Rep 2018;8:6220. [Crossref] [PubMed]
- Tang X, Shu Z, Zhang W, et al. Clinical significance of the immune cell landscape in hepatocellular carcinoma patients with different degrees of fibrosis. Ann Transl Med 2019;7:528. [Crossref] [PubMed]
- Mizukoshi E, Kaneko S. Immune cell therapy for hepatocellular carcinoma. J Hematol Oncol 2019;12:52. [Crossref] [PubMed]
- Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453-7. [Crossref] [PubMed]
- Wang H, Huo X, Yang XR, et al. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer 2017;16:136. [Crossref] [PubMed]
- Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [Crossref] [PubMed]
- Diboun I, Wernisch L, Orengo CA, et al. Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genomics 2006;7:252. [Crossref] [PubMed]
- Liebner DA, Huang K, Parvin JD. MMAD: microarray microdissection with analysis of differences is a computational tool for deconvoluting cell type-specific contributions from tissue samples. Bioinformatics 2014;30:682-9. [Crossref] [PubMed]
- Abbas AR, Wolslegel K, Seshasayee D, et al. Deconvolution of blood microarray data identifies cellular activation patterns in systemic lupus erythematosus. PLoS One 2009;4:e6098. [Crossref] [PubMed]
- Zhong Y, Wan YW, Pang K, et al. Digital sorting of complex tissues for cell type-specific gene expression profiles. BMC Bioinformatics 2013;14:89. [Crossref] [PubMed]
- Chen B, Khodadoust MS, Liu CL, et al. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol 2018;1711:243-59. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Foerster F, Hess M, Gerhold-Ay A, et al. The immune contexture of hepatocellular carcinoma predicts clinical outcome. Sci Rep 2018;8:5351. [Crossref] [PubMed]
- Hsiao YW, Chiu LT, Chen CH, et al. Tumor-Infiltrating Leukocyte Composition and Prognostic Power in Hepatitis B- and Hepatitis C-Related Hepatocellular Carcinomas. Genes (Basel) 2019;10:630. [Crossref] [PubMed]
- Thompson ED, Enriquez HL, Fu YX, et al. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med 2010;207:1791-804. [Crossref] [PubMed]
- Garnelo M, Tan A, Her Z, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017;66:342-51. [Crossref] [PubMed]
- Shi JY, Gao Q, Wang ZC, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res 2013;19:5994-6005. [Crossref] [PubMed]
- Sung PS, Jang JW. Natural killer cell dysfunction in hepatocellular carcinoma: pathogenesis and clinical implications. Int J Mol Sci 2018;19:3648. [Crossref] [PubMed]
- Cai XY, Gao Q, Qiu SJ, et al. Dendritic cell infiltration and prognosis of human hepatocellular carcinoma. J Cancer Res Clin Oncol 2006;132:293-301. [Crossref] [PubMed]
- Ninomiya T, Akbar SM, Masumoto T, et al. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol 1999;31:323-31. [Crossref] [PubMed]
- Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007;132:2328-39. [Crossref] [PubMed]
- Kuang DM, Zhao Q, Wu Y, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol 2011;54:948-55. [Crossref] [PubMed]
- Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 2011;54:497-505. [Crossref] [PubMed]
- Ji J, Eggert T, Budhu A. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology 2015;62:481-95. [Crossref] [PubMed]
- Liu RX, Wei Y, Zeng QH, et al. Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology 2015;62:1779-90. [Crossref] [PubMed]
- Li JQ, Yu XJ, Wang YC, et al. Distinct patterns and prognostic values of tumor-infiltrating macrophages in hepatocellular carcinoma and gastric cancer. J Transl Med 2017;15:37. [Crossref] [PubMed]
- Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 2008;26:2707-16. [Crossref] [PubMed]
- Mossanen JC, Tacke F. Role of lymphocytes in liver cancer. Oncoimmunology 2013;2:e26468. [Crossref] [PubMed]
- Wu H, Chen P, Liao R, et al. Intratumoral regulatory T cells with higher prevalence and more suppressive activity in hepatocellular carcinoma patients. J Gastroenterol Hepatol 2013;28:1555-64. [Crossref] [PubMed]
- Zhang HH, Mei MH, Fei R, et al. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat 2010;17 Suppl 1:34-43. [Crossref] [PubMed]
- Li W, Han J, Wu H. Regulatory T-cells promote hepatitis B virus infection and hepatocellular carcinoma progression. Chronic Dis Transl Med 2016;2:67-80. [PubMed]
- Tu JF, Ding YH, Ying XH, et al. Regulatory T cells, especially ICOS(+) FOXP3(+) regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep 2016;6:35056. [Crossref] [PubMed]
- Sun L, Xu G, Liao W, et al. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: a meta-analysis. Oncotarget 2017;8:39658-72. [Crossref] [PubMed]
- Wang Y, Liu T, Tang W, et al. Hepatocellular Carcinoma Cells Induce Regulatory T Cells and Lead to Poor Prognosis via Production of Transforming Growth Factor-beta1. Cell Physiol Biochem 2016;38:306-18. [Crossref] [PubMed]
- Shi C, Chen Y, Chen Y, et al. CD4(+) CD25(+) regulatory T cells promote hepatocellular carcinoma invasion via TGF-beta1-induced epithelial-mesenchymal transition. Onco Targets Ther 2018;12:279-89. [Crossref] [PubMed]
- Juengpanich S, Shi L, Iranmanesh Y, et al. The role of natural killer cells in hepatocellular carcinoma development and treatment: A narrative review. Transl Oncol 2019;12:1092-107. [Crossref] [PubMed]
- Chen Y, Hao X, Sun R, et al. Natural Killer Cell-Derived Interferon-Gamma Promotes Hepatocellular Carcinoma Through the Epithelial Cell Adhesion Molecule-Epithelial-to-Mesenchymal Transition Axis in Hepatitis B Virus Transgenic Mice. Hepatology 2019;69:1735-50. [Crossref] [PubMed]
- Zahran AM, Abdel-Meguid MM, Ashmawy AM, et al. Frequency and Implications of Natural Killer and Natural Killer T Cells in Hepatocellular Carcinoma. Egypt J Immunol 2018;25:45-52. [PubMed]
- Liu P, Chen L, Zhang H. Natural Killer Cells in Liver Disease and Hepatocellular Carcinoma and the NK Cell-Based Immunotherapy. J Immunol Res 2018;2018:1206737. [Crossref] [PubMed]
- Najima Y, Yoshida C, Iriyama N, et al. Regulatory T cell inhibition by dasatinib is associated with natural killer cell differentiation and a favorable molecular response-The final results of the D-first study. Leuk Res 2018;66:66-72. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)